Ali Discusses Treatment Options and Spleen Size in Primary Myelofibrosis
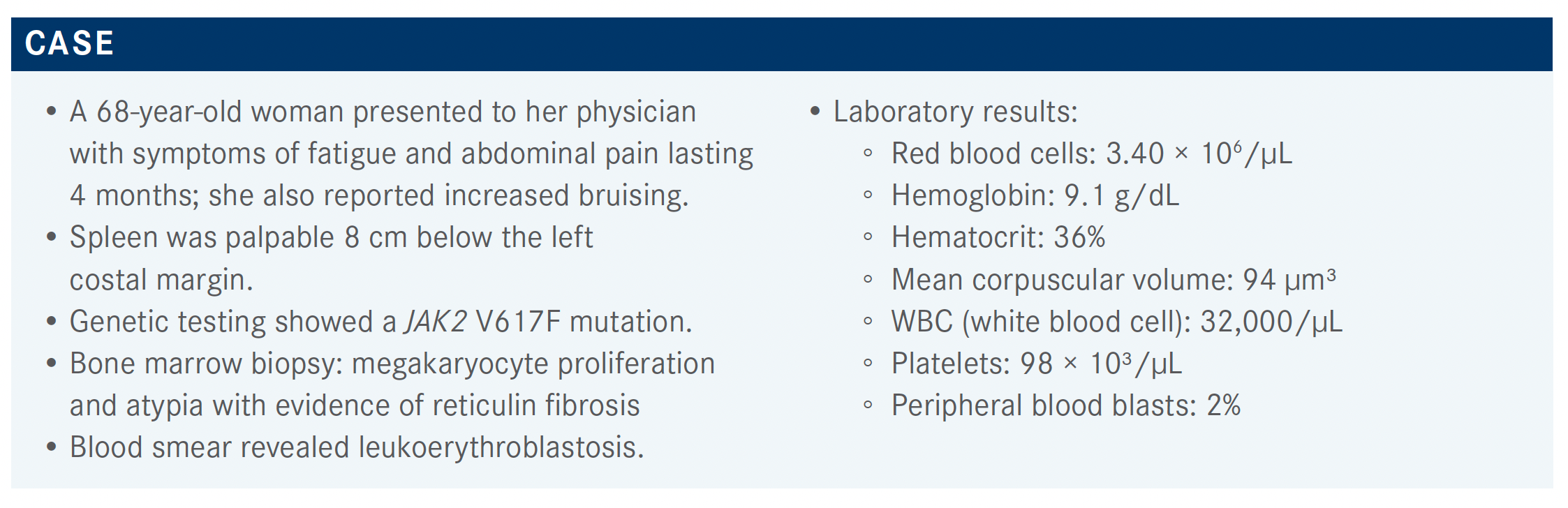
Haris Ali, MD, discussed a 68-year-old woman who presented to her physician with symptoms of fatigue and abdominal pain lasting 4 months; she also reported increased bruising.
Haris Ali, MD

During a Targeted OncologyTM Case-Based Roundtable event, Haris Ali, MD, associate clinical professor, Department of Hematology and Hematopoietic Cell Transplantation at the City of Hope, discussed a primary myelofibrosis patient case.
Targeted OncologyTM: What are the differences among the various prognostic risk models for myelofibrosis?
ALI: The International Prognostic Scoring System [IPSS], the Dynamic IPSS [DIPSS], and the DIPSS-plus are all validated prognostic models for primary myelofibrosis. For the IPSS and DIPSS, they use the same parameters [older than 65 years, hemoglobin level lower than 10 g/dL, white blood cell count greater than 25 × 103/μL, peripheral blood blasts 1% or higher, and the presence of constitutional symptoms]. For DIPSS-plus, however, it’s more comprehensive, as it includes unfavorable karyotype transition, red blood cell transfusion dependency, and low platelet count [less than 100 × 103/μL]. Using these models, the median survival of the risk groups is as follows: low-risk survival is [greater than] 11 years, intermediate-risk-1 survival is 6 to 14 years, intermediate-risk-2 survival is 3 to 4 years, and high-risk survival is 1 to 2 years.1-3
The MIPSS70 model [Mutation-Enhanced IPSS] is a little more recent [2018]. In addition to previously described variables, it also incorporated these variables: bone marrow fibrosis grade of 2 or more, absence of CALR type 1 mutation, and presence of high-molecular risk [HMR] mutations, where greater weight is given to the presence of 2 or more mutations. Mutations in the HMR category include ASXL1, EZH2, SRSF2, IDH1/2, and U2AF1. Low-risk average survival is [greater than] 2 decades, [whereas] intermediate-risk [average] survival is 7 years, and high-risk [average] survival is 2.3 years.4
The most recent model is the MIPSS70-plus version 2.0, in which the authors used the latest categories for cytogenetics. They divided the groups into 5 risk categories: very low, low, intermediate, high, or very high. Median overall survival [OS] ranged from median not reached at very low risk to 1.8 years at very high risk. This was validated in the Mayo Clinic cohort, as well as in an Italian cohort. The very high-risk karyotypes include monosomy 7, isochromosome 17, inversion of chromosome 3, abnormalities of 12p or 11q, and autosomal trisomies not including +8 or +9.5,6
A prognostics scoring system was developed by the Italian group called MYSEC-PM [Myelofibrosis Secondary to PV (polycythemia vera) and ET (essential thrombocytopenia)-Prognostic Model], which uses age, hemoglobin, platelets, circulating blasts, CALR, and constitutional symptoms. It was validated for secondary myelofibrosis and survival. For high-risk survival, it’s... close to 2 years and for low risk, median is not reached, and intermediate-1 and intermediate-2 are in-between.7
The updated National Comprehensive Cancer Network [NCCN] guidelines recommend checking 1 of the prognostic models for patients: the MIPSS, DIPSS, or MYSEC. They stratify to 2 different risk groups, lower risk or higher risk, depending on the score.8
What are the NCCN guidelines for managing high-risk myelofibrosis?
If you look at the NCCN guidelines for high-risk myelofibrosis, the recommendation is to assess the symptom burden using 1 of the symptom assessment forms; MPN-10 is probably the simplest one. It has only 10 questions and is easily scalable. Patients who have low platelets do poorly, so if they are transplant candidates, you proceed to transplant. If they’re not candidates, they can be considered for clinical trials. Patients with platelet counts [greater than] 50,000 mm3, because they’re high risk, can be considered for transplant. If they’re not transplant candidates, the options are JAK inhibitor therapy, clinical trial, and then monitor symptoms and monitor response. If they are responding, continue treatment. If they are not responding, then they can go on an alternative JAK inhibitor or a clinical trial. If the patient progresses to acute myeloid leukemia or advanced myelofibrosis, they’re treated accordingly. Still, if transplant is an option, that can be considered. If a patient is not a transplant candidate and has symptomatic anemia, that can be treated a little bit differently.8
Can you describe the response to ruxolitinib in the COMFORT trials?
There were 2 COMFORT trials that led to the approval of ruxolitinib [Jakafi] in 2012, the first drug that came to the market for myelofibrosis. The COMFORT-I trial [NCT00952289] was a United States–based trial, which was a randomized trial between ruxolitinib and placebo.9 The COMFORT-II trial [NCT00934544] was mainly a European trial that compared ruxolitinib with the best available therapy.10
In the COMFORT-I trial, 41.9% of patients met the primary end point, which was greater than 35% spleen volume reduction [SVR] vs 0.7% of patients in the placebo group. The majority of the patients on the ruxolitinib had a response; even if they did not reach the primary end point, most of the patients did have reduction in spleen size.9 Similarly, in the COMFORT-II trial, 28% of patients taking ruxolitinib met the same primary end point and the majority of patients had a spleen volume response. Overall, 97% of the patients had a SVR vs 56% of the best available therapy [BAT] arm. A very small percentage of the patients had spleen enlargement on the preterm compared with the BAT.10
Looking at the symptom response in COMFORT-1, the secondary end point was more than 50% improvement in the total symptom score. The majority of the patients had more than 50% reduction compared with a very small percentage of patients in the placebo arm. Similarly, there was improvement in the individual symptoms with ruxolitinib vs placebo. If you look at the onset of symptoms, they improved within 4 to 6 weeks. We don’t have this kind of response for the spleen volume, but the symptoms improved dramatically between 4 and 6 weeks.9 For the COMFORT-II trial, they used a different symptom assessment tool, but, again, there were better responses in all the symptoms in the ruxolitinib arm vs the BAT arm.10
They also presented survival data. Compared with the control arms, in both the COMFORT trials, patients who were on ruxolitinib had improved survival, and this was significant. [Median OS was 5.3 vs 3.8 years (HR, 0.70; 95% CI, 0.54-0.91; P < .001)].11 There was a retrospective study by Cervantes et al in 2009 that looked at causes of death, including transformation to acute leukemia, primary myelofibrosis progression without acute transformation, thrombotic events, infection, bleeding, portal hypertension, or other causes.1 This included [approximately] 1000 patients. Median survival was 69 months, and there were [approximately] 49% of the patients who died on the analysis.1
Looking at the effect of the spleen response on OS, patients who have a spleen response with 35% volume reduction have a longer survival compared with those patients who don’t have that spleen response. Also, patients who have more durable response, their survival is much greater.12 Baseline factors associated with lower response include patients who have a high-risk disease with a very big spleen more than 20 cm below the costal margin, very high white [cell] count, delaying starting ruxolitinib after diagnosis, or keeping it at a lower dose rather than escalating the dose.13,14
They also looked at the various doses and their responses. Patients on a lower dose—for example, less than 10 mg twice daily—had a lower response compared with patients who were on a higher dose, both for symptoms and for spleen volume. At 10 mg twice daily or more, you had a better response. It’s better to avoid starting with the lower dose, but if you start low, you should then escalate quickly to the maximum safe dose. Doses less than 10 mg twice daily may not be very effective for the long term.15
Looking at grade 3 to 4 adverse events [AEs] in both the COMFORT-I and COMFORT-II trials, anemia and thrombocytopenia were prominent, which can be explained by the mechanism of action from inhibiting the JAK pathway.9,10
What do you think of the dosing for ruxolitinib?
A study called EXPAND [NCT01317875] was done to assess how high a dose of ruxolitinib could be tolerated in patients with thrombocytopenia. Patients who had moderate thrombocytopenia, with platelet counts of 75 to 99 [× 103/μL], were started at 5 mg in the morning and 5 mg in the afternoon, and doses were gradually escalated to 15 mg twice daily. Once they saw that there were not too many AEs, they included these doses for the patients with platelet counts of 50 to 74 [× 103/μL]. A maximum safe dose was established at 10 mg twice daily in both arms. Thrombocytopenia and anemia were still observed in these patients. Even though the platelet count was low, patients still benefited from the treatment, with SVR and improvement in the symptom score.16
thiamine [vitamin B1]. That’s why these patients required thiamine to be checked prior to the treatment, replaced, and then monitored during the treatment.17
If you look at the high-risk myelofibrosis algorithm [NCCN guidelines], if the patient’s a transplant candidate, they can go to transplant. If they’re not a candidate, assess symptoms. Then depending on the platelet count, if it’s below 50 [× 103/μL], they’re not candidates for ruxolitinib or fedratinib; they can go on the trials. If it’s over 50 [× 103/μL], then they can go on any other JAK inhibitor. Fedratinib is also added now [because] it was approved. You follow the paradigm of following response and continuing treatment if the patient is responding. If they’re not responding, then consider changing treatment.8
Do you use splenectomy in your treatment?
Very few patients will benefit from splenectomy. Occasionally, you will have a patient who has very low-risk disease and has failed a JAK inhibitor where the spleen is still enlarged. Then I would consider doing splenectomy. Most of the time they will be patients who are CALR mutation positive. They don’t get the best response to JAK inhibitors. A couple of patients were falling into very low-risk [group] by the MIPSS and DIPSS criteria; they were symptomatic, they had anemia. So I took out the spleen and the platelets improved, the hemoglobin improved, and their lives improved tremendously.
For patients who are transplant candidates and either are not responding to JAK inhibitors or have a low platelet count, would you take them to transplant without a splenectomy?
There’s a recent very good study that came out of Europe [with results that] explained how spleen size and splenectomy...affect transplant. In this patient who had a spleen size of [greater than] than 20 cm, they had a really hard time after transplant with cytopenia. Either you can consider doing a myeloablative regimen if they’re eligible, rather than doing the reduced intensity, or if the patient has otherwise good performance status, you can consider doing a splenectomy. It was a retrospective study, but that means that’s something that they saw; 20 cm was the cutoff point for outcome change.
REFERENCES:
1. Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895-2901. doi:10.1182/blood-2008-07-170449
2. Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More