Roundtable Discussion: Udhrain Discusses Treatment Management in Patients With Extensive-Stage SCLC
In a 73-year-old patient, a PET scan revealed activity in the left upper lobe mass, supraclavicular nodal areas, and liver lesions, and an MRI discovered 1 small asymptomatic brain lesion. This lead to a diagnosis of stage T3N3M1-IV extensive-stage small cell lung cancer.
Ashish Udhrain, MD


During a Targeted OncologyTM Case-Based Roundtable, Ashish Udhrain, MD, medical oncologist, Thibodaux Regional Health System in Thibodaux, LA, discussed the case of a 73-year-old patient with extensive stage small cell lung caner with a group of peers.
RESKE: Long-term survival [is one of the biggest unmet needs]. Initially, the patient responds well with immunotherapy and chemotherapy, [and] the cancer melts away. But then it comes back with a vengeance.
Even with the new second-line treatments available, I feel once the patient progress, it’s really a challenging disease and they end up in hospice or die.
UDHRAIN: That’s a great point. Believe it or not, the first published paper on SCLC treatment with cisplatin [Platinol] and VP-16 [etoposide (Toposar)] was published in the JCO [Journal of Clinical Oncology] in 1985.1
Fast forward to now, well that’s how little advancement we’ve made in the treatment of this disease. Unfortunately, you’re right in that regard.
FEAGIN: For me, the challenge is patients not being fit enough for second- or third-line options and even having reasonable options for them.
UDHRAIN: Most of the time, especially in this disease state, performance status and quality-of-life discussions become more prudent.
SCHAEFER: I routinely have problems with brain metastases. [Patients] get brain metastases and [are] treated, but they always recur.
Like you said, that really affects [patients’] quality of life and their ECOG performance status. Right now, a lot of times I can control [metastases] well viscerally, but once [they get] in the brain, it’s just hurdle after hurdle.
UDHRAIN: What are the most common barriers to not using immunotherapy in this setting?
RESKE: I feel like if someone has an autoimmune disease, it’s for sure where I’m hesitant.
UDHRAIN: I had a patient with bad lupus that I could not use immunotherapy on, certainly. So I absolutely agree with that.
PATEL: Insurance coverage may be an issue for some patients with high deductible plans.
UDHRAIN: I think more of us are dealing daily with prior authorization and denials, etc, and are having to appeal for our patients. That’s certainly becoming the problem for all of us practicing now.

BAGHIAN: I don’t know of any data to support waiting, especially with the behavior for SCLC. It’s on the aggressive side. Usually those are the patients [for whom] I’m really racing against the clock because every second counts to treat them.
UDHRAIN: I’ve had some colleagues send for NGS testing for clinical trial purposes. Outside of that, I think as soon as the diagnosis of SCLC is made, everybody is on the clock to get started on the treatment as quickly as possible.
BAGHIAN: I’m not aware of any data to support the correlation between PD-L1 expression and the use of atezolizumab [Tecentriq] unless I’m mistaken.
UDHRAIN: No, you’re right. In the clinical trials that was not a requirement, but they checked it. I think they were collecting data on the back end of it, but it was not a stratification requirement.
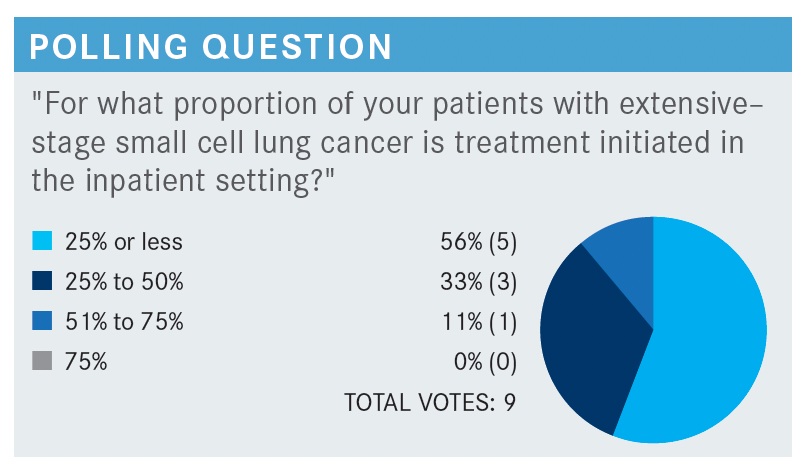
UDHRAIN: There’s a discussion at least in my practice that cisplatin and VP-16 is relatively cheap to give in the inpatient setting and less hard to convince the hospital administration to pay for.
Now with the curveball of not adding immunotherapy, it’s become a financial challenge from that standpoint, at least in my practice. I’m open to hear what’s going on in your institutions or the hospitals that you’re affiliated with regarding inpatient treatment.
SCHAEFER: The only time now that we will ever do inpatient is someone who’s on a ventilator or is hemodynamically unstable.
We use just the cytotoxic agents in the hospital. The hospital I work at is kind of like yours. They refuse to do it, and I am not a hospital employee, so it seems a little more onerous to try to get IO [immuno-oncology] therapy if you need it.
Again, if someone’s unstable and needs to get off the ventilator or some other reason, we’ll do it. But if not, it is done almost 100% of the time as an outpatient.
FEAGIN: If we choose to do inpatient, for some reason the immunotherapy must wait until the second cycle. They will not approve it for inpatient.
UDHRAIN: It’s become pretty much nationwide, and all of us face the same situation, no matter where we’re treating our patients. In my practice as well, if I must give it inpatient, I’ll give cisplatin or carboplatin plus VP-16, and I’ll use the IO agents for the second cycle in the outpatient setting.
BAGHIAN: I remember back at university or in charity the patients would come in so sick and debilitated [that] we would treat them commonly in the hospital setting.
Is this the kind of disease where you would treat a patient even with ECOG performance score [PS] 3? Would you support the patient with that ECOG PS and kind of bend those rules?
UDHRAIN: I would because if you compare with diffuse large B-cell lymphoma, for example, where patients come in severely debilitated from the disease, but I give at least one cycle to support them and see if their symptoms improve. Obviously, if they have declining status despite that or if they just have uncontrolled brain metastases that just keep causing more issues, then at that point, it becomes a quality-of-life discussion with the patient and the family members. If there’s no response, progression, or deterioration of the performance status, that’s when I tend to back off. But I give at least one cycle a try.
BAGHIAN: I don’t know if we’re going to cover this, but would you concurrently treat the brain metastases with SRS [stereotactic radiosurgery] during the induction phase?
UDHRAIN: If [the brain metastases are] symptomatic, and depending on how many they are, I don’t mind SRS. I’ve done that with good outcomes and good responses, but if it’s asymptomatic, they should do fine. For oligometastases or isolated metastases, I kind of wait to see how systemic response will be or I treat that first before starting.
MERRICK: The patient had brain metastases, and [it’s] always really challenging on how to treat ES-SCLC with brain metastases and which modality to try to prioritize. Generally, the algorithm I look at is if the patient is symptomatic, or are there clinical ramifications to the brain metastases? If there’s midline shift and the patient is neurologically compromised, I tend to emphasize getting the brain metastases under control first.
If these are small, asymptomatic brain metastases that are found incidentally on a staging MRI, then I prioritize systemic treatment and complete induction systemic therapy, whether it’s chemotherapy alone or chemo-immunotherapy, and monitor the patient. If the brain metastases are stable or responding and you get an MRI every 2 cycles or so, just push ahead with systemic treatment. After the systemic induction treatment is completed, then one can address the brain metastases with SRS, whole brain radiotherapy, or whatever else is indicated.
UDHRAIN: In the case study, the patient had 1 isolated asymptomatic brain metastasis.
So now the discussion would be first and foremost for ES-SCLC to begin treatment with systemic therapy in this patient. I am curious how many of you use durvalumab [Imfinzi] vs atezolizumab in combination with cytotoxic therapy?
BAGHIAN: Atezolizumab came out first, so I use that.
FEAGIN: I also use atezolizumab.
SCHAEFER: The other thing that impacts [this decision] is durvalumab. At least for us, it has a slightly better drug replacement program, so the drug gets replaced right away if [the patient] can’t get coverage.
UDHRAIN: Now that the payors are becoming their own PBMs [pharmacy benefit managers] or are buying out PBMs, they’re creating their own preferred list in all of these. I’ve heard that at some state levels, as well as at [American Society of Clinical Oncology], there have been issues and discussions about how payors are dictating which drug they prefer, which I find very interesting.
The [National Comprehensive Cancer Network] guidelines version 2021 for ES-SCLC recommends 4 cycles of chemotherapy, but some patients may receive up to 6 cycles depending on tolerability.
The preferred regimens are 4 cycles of carboplatin, etoposide, [and] atezolizumab, followed by atezolizumab maintenance; carboplatin, etoposide, [and] durvalumab, followed by durvalumab maintenance; or cisplatin, etoposide, [and] durvalumab, followed by durvalumab maintenance.
PATEL: In metastatic SCLC, I will usually use carboplatin, rather than cisplatin, just because of the fewer adverse events [AEs] and very little change in prognosis.
UDHRAIN: I think that’s what I do in my practice too. For ES-SCLC, I tend to use more carboplatin rather than cisplatin just from a quality-of-life and tolerability standpoint.
MERRICK: Has there ever been a head-to-head study comparing carboplatin with cisplatin in SCLC?
UDHRAIN: I’m not aware. I know there are data in non– small cell lung cancer [NSCLC], and the outcomes, even for the stage IV NSCLC, were pretty much the same.
MERRICK: Historically, the consensus was that for SCLC, cisplatin was a more efficacious agent, although certainly tolerability becomes an issue. But I’m not aware of any actual head-to-head comparison.
UDHRAIN: I’m not aware of any data in the extensive stage. I know there are a lot of the data in the limited stage, and we try to use as much cisplatin as possible concurrently with radiation.
MERRICK: Certainly, radiation can be given [concurrently], whether carboplatin is being administered or cisplatin is being administered. But from an efficacy standpoint, at least my understanding was that cisplatin was a bit more efficacious than carboplatin.
FEAGIN: One of the things that drives me to use carboplatin is the ease in giving it in the outpatient setting and the difficulty in giving immunotherapy as an inpatient. My tendency is to give carboplatin so I can keep it as an outpatient and go ahead and start the immunotherapy up front.

SCHAEFER: I have seen a problem with fatigue. And it’s not just in atezolizumab, but it’s really with any immunotherapy agents. I can tell you sometimes the fatigue is almost debilitating to patients.
Unfortunately, when that happens, there’s not a lot of reversing agents. I’m not talking about the fatigue, especially with the infusion. I’m talking about the long-term fatigue that can get patients who are post-IO therapy. We try different stimulants, but they don’t work, and even giving prednisone doesn’t help.
GILL: We had a patient who we thought developed [immune thrombocytopenic purpura] secondary to the atezolizumab. We are yet to rechallenge with the atezolizumab, as the patient presented with a platelet count of 10,000/μL, which we thought was due to atezolizumab. It was right after the second cycle.
BAGHIAN: Did you give steroids, and did the patient rebound after that?
GILL: Yes, we gave steroids, and there was complete resolution. But we haven’t rechallenged with atezolizumab, and I think honestly the patient [in this case] is about to go into hospice, so we won’t be able to find out.
UDHRAIN: I would say that’s almost publishable because I don’t think I’ve seen that anywhere.
BAGHIAN: I haven’t really had a lot of autoimmune AEs in patients with SCLC on atezolizumab.
UDHRAIN: Our case discussion patient developed diarrhea 3 to 4 times a day and nausea after his second cycle.
GILL: Well, in this case you look at the number of stool passage per day and how much that’s increased from baseline. For 3 to 4 times a day, that’s not that bad. It is still grade 1 because it’s fewer than 4 times. I would probably continue it or maybe hold temporarily and see how they do off it.
I would keep an eye on it and make sure that they don’t get dehydrated. If it gets any worse than that, of course we consider starting steroids and unfortunately pausing treatment or stopping treatment and consulting the GI [gastrointestinal] department.
UDHRAIN: I’ve started incorporating a lot of oncolytics, whether it’s oral or intravenous, and probiotics as a preventative measure from the standpoint of diarrhea, learning from my GI colleagues to kind of proactively prevent some of these symptoms. I’ve found some success with that from a supportive care standpoint.
REFERENCE:
1. Evans WK, Osoba D, Feld R, Shepherd FA, Bazos MJ, DeBoer G. Etoposide (VP-16) and cisplatin: an effective treatment for relapse in small-cell lung cancer. J Clin Oncol. 1985;3(1):65-71. doi:10.1200/JCO.1985.3.1.65
