Roundtable Discussion: Participants Compare Options in First- and Later-Line mCRPC
A 75-year-old man presented with intermittent right hip pain. The case was discussed between of physicians and moderated by Nauman Moazzam, MD.
Nauman Moazzam, MD


During a Targeted OncologyTM Case-Based Roundtable event, Nauman Moazzam, MD, medical oncologist/hematologist at the Rocky Mountain Cancer Centers in Lakewood, CO, discussed the case of a 75-year-old man with metastatic castration-resistant prostate cancer.
MUSHTAQ: I think now we can start antiresorptive medications, especially because the patient is castration resistant. I think the patient got the appropriate management with EBRT and 18 months of ADT.
SRIVASTAVA: So he became castration resistant 6 months after the radiation and he was on ADT then? That shows it’s probably a fairly aggressive disease, more aggressive than we would have thought.
MOAZZAM: Yes, within 6 months [he developed] metastatic disease.
SRIVASTAVA: So [this patient has] low-volume [disease].
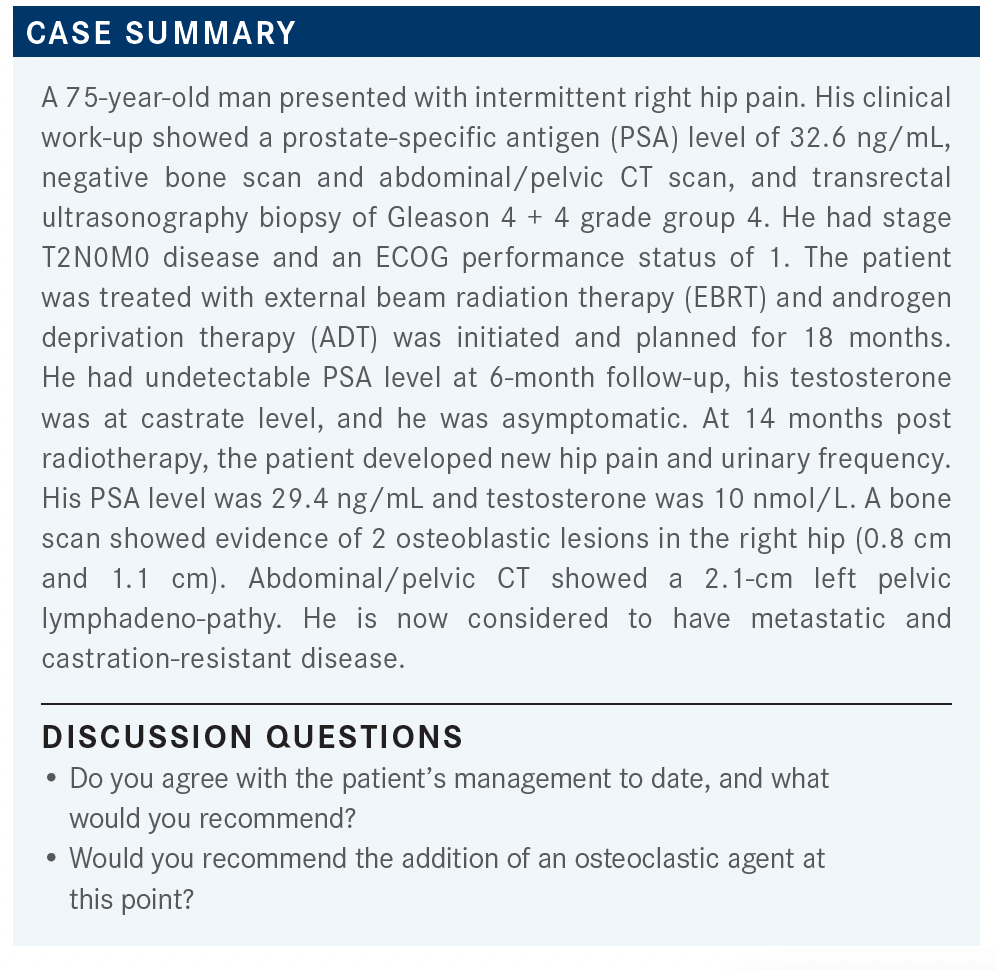
MOAZZAM: The NCCN [National Comprehensive Cancer Network] guidelines from February 2021 [say with] no prior docetaxel and no prior novel hormonal therapy, the category 1 recommendations are either abiraterone [Zytiga], docetaxel, or enzalutamide [Xtandi].1 Then, in some situations, you can use sipuleucel-T [Provenge] or radium 223 [Xofigo], or secondary hormonal therapy. Can anybody comment how they would choose between chemotherapy or AR-targeted therapy in this patient?
SORIANO: I chose enzalutamide because this is an elderly patient, and sometimes I’ve had issues using abiraterone plus prednisone in regard to tolerability, and [blood] sugar going out of control.
MOAZZAM: You would not use docetaxel because of the age?
SORIANO: No, I wouldn’t use docetaxel because it was 6 months [until] progression, but the extent of disease was basically 2 bony lesions and 1 pelvic lymph node. From my standpoint, I don’t think that’s significant [enough] to put him through chemotherapy.
MOAZZAM: There was a study published in Lancet in which they tried to sequence between abiraterone vs enzalutamide first.2 Do you have any preference whether, in the first-line setting, you use abiraterone or enzalutamide? In what sequence?
SRIVASTAVA: I think it really depends. If they’re diabetic, I may avoid abiraterone because of prednisone use. And [in the] elderly, with enzalutamide, they get a little bit of that central nervous system fogginess, and falls, so I may avoid that for that reason. In picking [between] those, I think it’s more tailored to the patient and adverse effect profile than anything else. In general, I don’t have a preference for 1 over another.
MOAZZAM: Are you aware of that study? It was a phase 2 trial in Lancet in 2019, that showed that people don’t respond that well to second-line abiraterone compared with enzalutamide.2 So the authors concluded abiraterone should be used first line, followed by enzalutamide, because in the crossover trial people who got enzalutamide first did not respond to abiraterone that much.
VICUNA: Yes, that’s what I base my sequencing on. I use abiraterone first before enzalutamide. I know the prednisone can be an issue in older patients. I sometimes don’t give them prednisone up front unless they start to have swelling. It’s really only for edema, and most patients seem to tolerate abiraterone even without the prednisone.
FAHED: Abiraterone is now generic, way cheaper, and the price will keep going down. I don’t see any reason why we should not use it first line, so a patient will be on it for a year or more.
KARSH: I think it depends on the patient. I think abiraterone is very well tolerated. Especially in some older patients, I think that enzalutamide sometimes creates more cognitive function issues. They can have more falls. So unless they have a medical issue that would preclude them from abiraterone, I like to start with abiraterone.
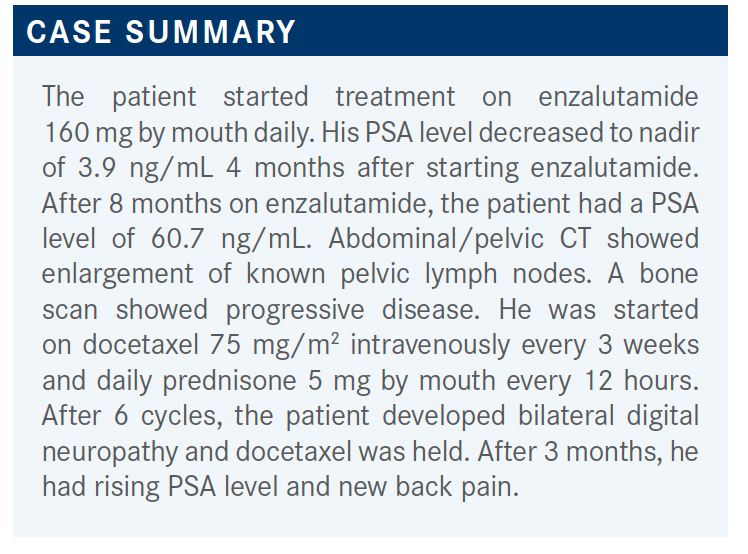
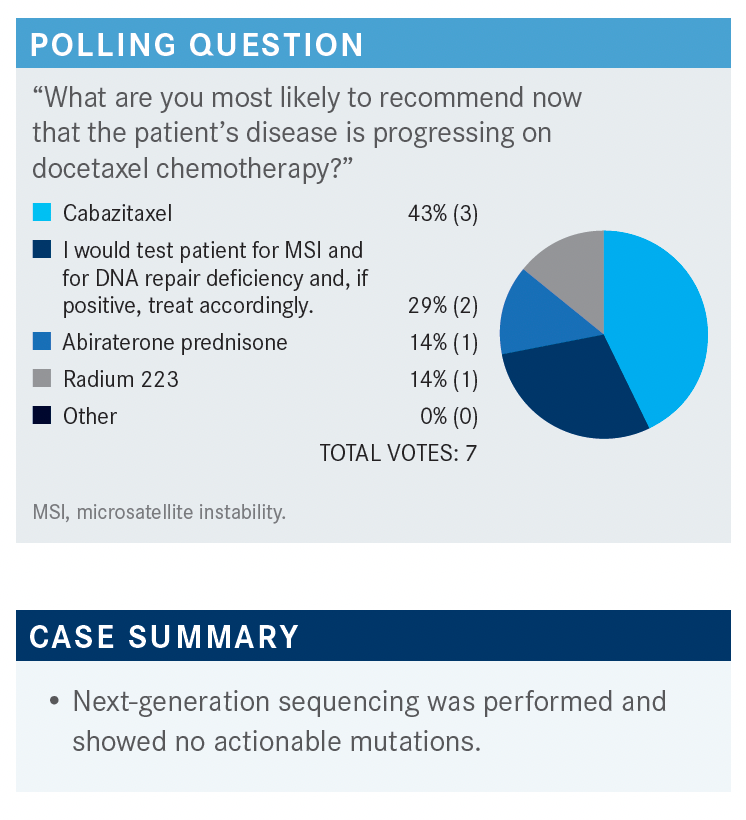
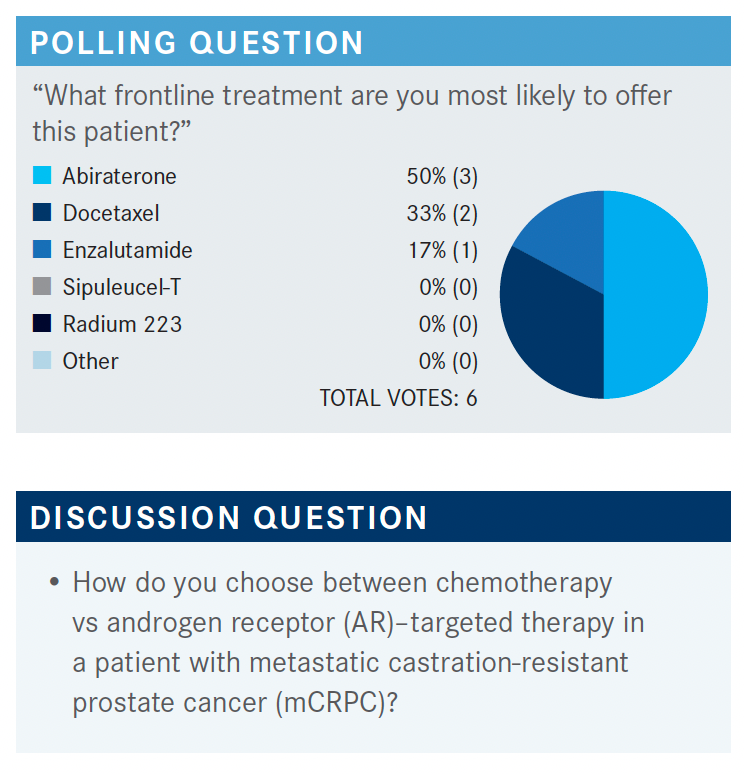
MOAZZAM: For this case, next-generation sequencing was performed, and there were no actionable mutations. [We can look at options from] the NCCN guidelines for the patient with prior docetaxel chemotherapy, as well as prior novel hormonal therapy. The options are to use cabazitaxel [Jevtana] or [rechallenge docetaxel, but] I don’t rechallenge docetaxel unless it was used years ago, and it’s still [having an affect].1 Then, in certain situations, you can use olaparib [Lynparza], but the patient was negative for any mutations. I personally have never used cabazitaxel with carboplatin at the same time; pembrolizumab [Keytruda], the case was not microsatellite instability high so we cannot use that; radium 223, but the patient was not that symptomatic in this situation right now.

KARSH: I would probably consider cabazitaxel. He progressed pretty rapidly after docetaxel so I don’t think that’s going to work, and he developed neuropathy on it.
I think that considering cabazitaxel would not be a bad choice here. You could check for AR-V7, because sometimes if they’re AR-V7–positive, and they get a taxane, they can revert to AR-V7–negative. You could consider an oral agent again, in that sense. He was on enzalutamide; put him on abiraterone. But I think I would probably consider cabazitaxel.
HINSHAW: I would not go to a second hormonal therapy in this situation; I would use the chemotherapy. In fact, I even use mitoxantrone in clinical practice. I know that fell out of favor with the newer drugs, but I still have patients who respond to mitoxantrone as well.
MOAZZAM: Would you use mitoxantrone before cabazitaxel or after?
HINSHAW: After; it’s like my third-line chemotherapy.
MOAZZAM: I have used mitoxantrone. Exactly like you: If somebody’s progressing and I’ve used every single thing, and there’s no clinical trial available, and there’s no mutations, and the patient has a decent performance status, I use mitoxantrone.
MOAZZAM: Is anybody testing for the AR-V7 splice variant?
HINSHAW: I don’t.
FAHED: I tested once or twice for AR-V7. The problem, a lot of times, is that’s the only option you have. I think cabazitaxel is much better tolerated than docetaxel. There was a question if it’s a different agent, or there’s efficacy similar to rechallenging with docetaxel. But, in my practice at least, my experience has been that cabazitaxel is much better tolerated. The majority of those patients are older gentlemen, frail in a way, and docetaxel is really hard on them. I had a better experience with cabazitaxel.
KARSH: If I didn’t have a clinical trial, I think [I would use] cabazitaxel. I think there’s some evidence from the CARD trial [NCT02485691] that it might not be a bad option at this point.3,4

SRIVASTAVA: I think even before CARD, cytopenia was a concern, but the FDA labeling also changed, and the dose came down. I think it was 25 mg/m2, now from 25 to 20 mg, so that helped.5 I find some patients do have a bit more beaten-up marrow, or large, bony metastases, and that affects the marrow, too. So I may reduce the dose in the case of some cytopenias if they’re coming to fourth line and don’t have a robust marrow. Otherwise, if they’ve had docetaxel, and they have neuropathy, you might adjust the dose, but sometimes you really don’t have an option. So those 2 are the main issues I’m faced with: cytopenias and neuropathy.
KARSH: Are you seeing less toxicity with the lower dose of cabazitaxel now? In the CARD trial, it was 25 mg.3,4 Now it’s approved to use 20 mg, and I don’t know if that has improved toxicity. I remember when the TROPIC trial [NCT03356912] came out, when they compared docetaxel to cabazitaxel, the big thing was febrile neutropenia, and that seems to scare people a little bit.6
MOAZZAM: Right, they’re seeing less; plus, we are using Neulasta [pegfilgrastim] as growth factor support right away up front.
MUSHTAQ: Yes, granulocyte colony-stimulating factor is recommended with a 25-mg dose.
MOAZZAM: Before that we were not using it, and now I think nobody orders cabazitaxel without long-acting Neulasta or something like that.
SORIANO: So far, I haven’t had too many issues [with toxicity]. I don’t have too many patients on cabazitaxel, but [with] the ones I’ve had, cytopenia is usually the main issue. The neuropathy hasn’t gotten significantly worse. So other than the cytopenias, which are managed now with Neulasta, [there] hasn’t been a problem.
VICUNA: I agree. I think that probably the only issue can be neuropathy, and the Neulasta support has helped.
MUSHTAQ: Is everybody using 20 mg, or [do] people choose between 25 and 20 mg based on disease biology and patient comorbid conditions?
MOAZZAM: I start with 25 mg if it’s a younger patient and I feel they can tolerate it. If it’s a very elderly patient, I go to 20 mg starting dose. But at least try to give, first cycle, 25 mg and see how they do.
KARSH: It looked like they were getting a better quality of life with cabazitaxel than the oral agent. I just wonder if they’re getting better treatment for their cancer by giving them a more potent therapy.
MOAZZAM: The quality of life was bad at baseline because of disease progression. So once you controlled the disease rapidly, despite the chemotherapy being more toxic than either enzalutamide or abiraterone, their overall quality of life was better. That was my read from the trial.7,8
FAHED: Are you still using 25 mg, even after the FDA lowered the approved dose to 20 mg? Anybody else change their practice?
VICUNA: Yes, I have now that there’s an option for 20 mg, especially. But I base it on the patient’s performance status, comorbidities, and age. If I think they can tolerate it, I might give them 25 mg; but most of the time, I tend to have reduced the dose already. Because they’ve been beaten up already by docetaxel prior [to this].
MOAZZAM: I think [with the] majority of my patients I start at 20 mg, because they’re elderly patients, they’re heavily pretreated, and they’ve all received 8 cycles of docetaxel. I don’t know of the head-to-head trial between 25 and 20 mg, but I’m just seeing if they can get more drug, if they’re young and can tolerate it. And you can always go down.
FAHED: I don’t think I’ve used the 25 mg since the FDA changed the label, but I wonder for some patients who are really tolerating it well, [if we] should try to step up therapy.

MUSHTAQ: I think my [reasons in] primarily going for intravenous [IV] would be the disease biology, aggressiveness, and not responding to earlier line of therapy, with acceptable toxicity, and also supportive care to manage those toxicities. So that’s how I try to approach the patient.
MOAZZAM: With this question, what we’re trying to answer is, “Will the patients argue with you between an oral drug vs IV?” In my practice, as long as I tell the patient their disease is progressing, and they need IV therapy, I think patients don’t argue too much. That’s what my feeling is.
FAHED: My experience [is that] after trying both docetaxel and, let’s say, abiraterone, they really want an oral as
their next step. Because they associate chemotherapy with docetaxel, and they associate oral therapy with prior experience, and they’re really beaten down by docetaxel. Sometimes it’s hard to convince them to try another chemotherapy.
KARSH: I think it’s very important how you present it to the patient. I guess if you have a urologist that’s sitting there, they may try to sway them more toward the oral, vs an oncologist. Patients have a stigma about chemotherapy. But I think if you present it to them that they’re going to get better treatment with chemotherapy than another oral agent, and [it] will halt their progression a little better, most patients will go for the better treatment. I think it’s all in how it’s presented.
MOAZZAM: Those quality-of-life data were very impressive in the CARD trial.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 1.2022. Accessed September 27, 2021. https://bit.ly/3uadtsO
2. Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20(12):1730-1739. doi:10.1016/S1470-2045(19)30688-6
3. de Wit R, Kramer G, Eymard JC, et al. CARD: randomized, open-label study of cabazitaxel (CBZ) vs abiraterone (ABI) or enzalutamide (ENZ) in metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2019;30(suppl 5):v851-v934. doi:10.1093/annonc/mdz394
4. de Wit R, de Bono J, Sternberg CN, et al; CARD Investigators. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506-2518. doi:10.1056/NEJMoa1911206
5. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35(28):3198-3206. doi:10.1200/ JCO.2016.72.1076
6. de Bono JS, Oudard S, Ozguroglu M, et al; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-54. doi:10.1016/S0140-6736(10)61389-X
7. Fizazi K, et al. Pain response and health-related quality of life (HRQL) analysis in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving cabazitaxel (CBZ) vs abiraterone or enzalutamide in the CARD study. Poster presented at: Genitourinary Cancers Symposium; February 13-15, 2020; San Francisco, CA. Abstract 16.
8. Fizazi K, Kramer G, Eymard JC, et al. Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): an analysis of a randomised, multicentre, open-label, phase 4 study. Lancet Oncol. 2020;21(11):1513-1525. doi:10.1016/ S1470-2045(20)30449-6
