Roundtable Discussion: Participants Explore Molecular Testing in the Lung Cancer Setting
A 59-year-old man presented with dyspnea on exertion, fatigue, anorexia, and a 5-lb weight loss. He received the diagnosis of stage IVA adenocarcinoma and had an ECOG performance status of 1.
Rachel E. Sanborn, MD


During a Targeted OncologyTM Case-Based Roundtable event, Rachel E. Sanborn, MD, the codirector of the Thoracic Oncology Program at the Earle A. Chiles Research Institute Providence Cancer Institute in Portland, Oregon, discussed the case of a 59-year-old man with lung cancer with 10 peers.
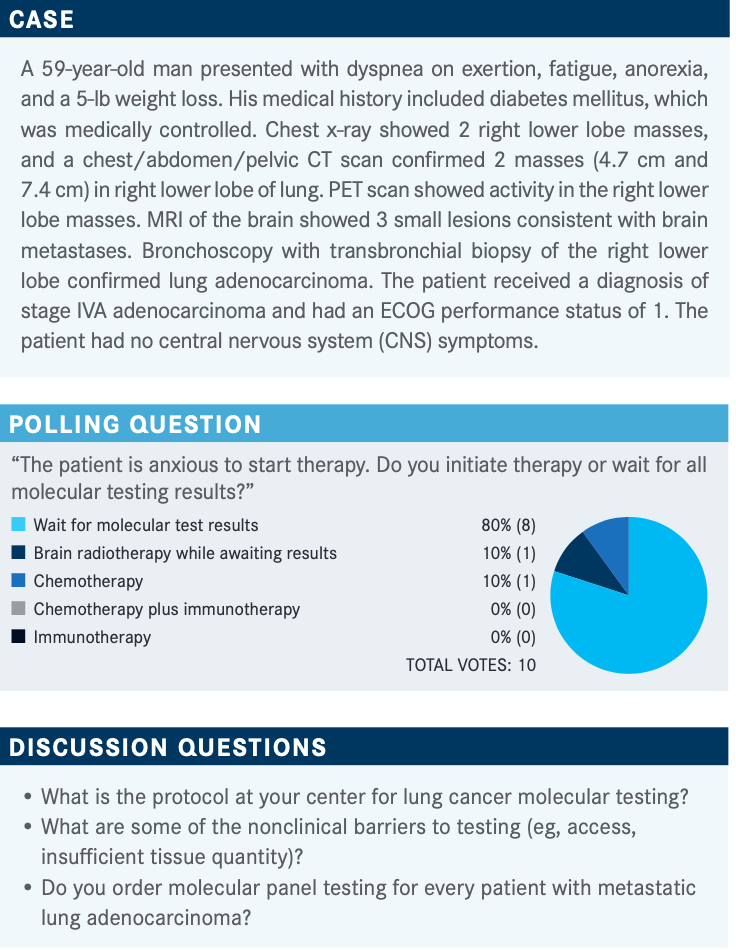
EY: I would do NGS [next-generation sequencing] on anybody with lung adenocarcinoma. Up until recently, it would just be EGFR, ALK, and ROS1, but now we’ve got more drugs for more mutations. There’s still some pushback on insurance, particularly toward multi-gene testing because that’s an easy target. Sometimes they’ll let you do [testing for] 2 or 3 mutations.
Getting [adequate] tissue and testing done on biopsies [are barriers too]. I recently had a case of an axillary metastasis, and it took very long to get it processed as specimen [for lung cancer].
SANBORN: Was it because of the biopsy of the axilla where they focus on RAS or something for that?
EY: Yes. I said, “Look, this is lung cancer, and this is an axillary metastasis,” and it didn’t go well. It worked out eventually, but it took a while. The NGS takes 2 weeks, so that’s always a challenge. It’s unlikely that a patient would need to be started on treatment sooner unless it’s a patient with a rare superior vena cava obstruction.
SANBORN: Is your testing run in-house?
EY: It depends on insurance. We do the solid tumor gene panel, which covers all the known mutations. I haven’t used FoundationOne for a while except if a trial requires it.
SUPERFIN: I order major mutations through the pathology departments and later do NGS, but it has so many mutations and actionable mutations. I do PD-L1 through NeoGenomics, and then I order Karius or FoundationOne NGS for the rest of the mutations.
SANBORN: Do you order these in all patients or only select ones?
SUPERFIN: I order in everyone if they’re diagnosed with stage IV lung adenocarcinoma.
KOSIEROWSKI: I work with the oncologists at the local prison and pretty much go along with whatever their protocol is. However, we certainly demand at least PD-L1, ALK, ROS1, and EGFR. I insist upon it for those patients with an atypical presentation and nonsmokers, though most of our patients are symptomatic at the time of presentation. It’s a different patient population.
SANBORN: What about things like MET exon 14 [METex14] skipping mutations and BRCA1/2?
KOSIEROWSKI: If the medical oncologist treating the patient orders one, if the patient is a nonsmoker, or if there’s a good clinical reason, then we will approve for advanced NGS testing. Otherwise, we just go along with the hospital protocol.
The major barrier is getting the drug from specialty pharmacies into the correctional setting because of security measures with outside drugs.
YANAGIHARA: In Hawaii, I routinely order comprehensive NGS testing on everybody with advanced nonsquamous lung cancer. The main problem in Hawaii has been that, until about 3 weeks ago, testing was not available locally, so we always had to send away. Because of the long turnaround time, I’ve always done the Guardant test first, which is a liquid biopsy. In select cases, if I don’t get an answer that I was expecting, I will do tissue NGS.
From the past 2 weeks, the tissue NGS is now available in Honolulu. The main barrier, though, is that they can only do 8 cases per week, so if you’re not part of those 8 cases, you are pushed to the next week and so on.
I do not routinely do PD-L1 testing because when I give chemotherapy, I’ll always give it with pembrolizumab [Keytruda]. I do not tend to give immunotherapy agents alone for patients not eligible for a targeted drug. I think for a while, I’ll still have to send away for the Guardant liquid biopsy, which has a turnaround time of 8 days in Hawaii.
SHAHIDI: I order Guardant first, and they now have a new service called TissueNext that automatically reflexes [to a tissue biopsy to perform additional genomic testing, including PD-L1 testing]. Sometimes I use FoundationOne too. I test all patients with advanced adenocarcinoma of the lung.
SANBORN: Does anybody have to separately order PD-L1 testing for patients with newly diagnosed lung adenocarcinoma, or is it just reflexed from pathology from the time of diagnosis?
CHEN: I think we specify the PD-L1 testing, so usually, we tell the pathology department to order the NGS as well as the PD-L1 testing.
ZHANG: I work in Washington state. They don’t do reflexed testing for PD-L1, but I think the biggest barrier for us is the tissue sample amounts. If you run out of tissue, then there’s not enough material to run NGS. Normally we use Karius testing, but for a patient with a big tumor burden, I send liquid biopsy samples as well. I’m trying to avoid further delays.

KOSIEROWSKI: Symptomatic patients need therapy, but this patient could probably sit and wait.
ZHANG: I agree. I think I would have all the chemotherapy ready to start but wait for the results. I would work on the chemotherapy beforehand because it takes another 2 weeks for the insurance company to approve and set up.
SANBORN: Two to 3 weeks is quite a delay in terms of waiting for the approval.
HARRIS: We tend to set up the chemotherapy regimen in advance because the patient must be scheduled. They must get approval through their insurance company. But I would not add the immunotherapy agent with the first cycle unless it’s BRAF-positive lung cancer and I’m not as worried about hyperprogression or something else that could go awry with adding immunotherapy up front. I don’t think you lose very much by holding on and waiting for the NGS testing results.
SHAHIDI: I don’t think anything is wrong with giving immunotherapy if the PD-L1 is 80%. Immunotherapy doesn’t work if you have EGFR or ALK mutations, but it works on RET or BRAF mutations. That’s my understanding.
SANBORN: What about the concerns from the TATTON study [NCT02143466] that showed if [a patient with an EGFR mutation] transitions from a checkpoint inhibitor to targeted therapy, they could have a higher incidence of immune toxicity with severe pneumonitis?1 Does that give you pause when thinking about that approach?
SHAHIDI: I would first wait to see whether the person has an EGFR mutation.

YANAGIHARA: In all the NGS reports from different companies that I’ve seen, you don’t have to read through the whole thing because they tell you which genes are altered. If it’s not mentioned in that top panel, then one can assume that all the others are not present.
KOSIEROWSKI: The one time I had problems with that, though, is with METex14-skipping mutations because that can be missed, especially on liquid tumor biopsies. You may need to do RNA testing to be sure of what you’re dealing with.
But it hasn’t posed a problem for me and my group so far because it’s a rare mutation. I don’t know if picking up MET amplification is a problem, but I know it is with the skipping mutation.
ZHANG: For NGS we use Karius here and normally, they’re very good at putting up a summary that is actionable. I recently saw a patient with an EGFR exon 20 insertion mutation. It’s an 8767-769 insertion mutation. They initially told me a second-generation TKI [tyrosine kinase inhibitor] would work. [The CHRYSALIS trial (NCT02609776) data supported the use of amivantamab-vmjw (Rybrevant) that was] newly approved in May 2021 by the FDA as frontline treatment for adult patients with non–small cell lung cancer [NSCLC] whose tumors have EGFR exon 20 insertion mutations.2
I ended up contacting the first author for the American Society of Clinical Oncology abstract. I wanted to know if he used osimertinib [Tagrisso] for some of the rare mutations; I think we still need a lot of guidance. Hopefully, in the future, we can have a platform with more lung cancer oncology experts that can give us some guidance.

YANAGIHARA: Very frequently—maybe 25% of the time. I think most of our lung cancer diagnoses are made by EBUS [endobronchial ultrasound]. When I get a QNS report, I just do liquid biopsy as the first step.
FEIN: It’s an issue that doesn’t need to exist. We infrequently have insufficient tissue. Anytime that I’m going in and doing a diagnosis and staging procedure, we have rapid-onset evaluation, and I rely heavily on the input of the cytopathologists who are present. Clearly, if what we’re dealing with is small cell lung cancer, I don’t need to make many passes. However, I recently had a patient who was intubated in the intensive care unit with locally advanced lung cancer in whom I made a total of 32 biopsy passes with a core needle.
There’s a learning curve with EBUS just like there is with anything. If, as a medical oncologist, you are frequently not getting what you need, then that’s the feedback that really needs to go to the pulmonologist that’s doing procedures. You shouldn’t have to deal with that issue. It doesn’t have to occur.
SUPERFIN: Most of our pathologists are sufficient. I rarely get insufficient analysis. I ask the surgeon in pulmonary who is good at getting us a good specimen. But I send liquid biopsy as well because some of the mutations, like EGFR, cannot be caught with tissue specimen.
ZHANG: I send [liquid and tissue testing] together. I don’t want to wait another 2 or 3 weeks and figure out there’s insufficient tissue, because there is a transition time between the different facilities. The turnaround time for liquid biopsy is shorter. It’s probably 1 week ahead of time, and sometimes that can be meaningful for some patients.
KOSIEROWSKI: If you’re sending those together, is insur-ance covering them?
ZHANG: The insurance would normally cover it, and for the ones they don’t cover, we have FoundationOne through the facility. Just like Tempus, they cover the cost.
SHAHIDI: I used to send Guardant and FoundationOne at the same time, but Guardant, which I now use, has a new program that reflexes to tissue NGS if the liquid biopsy is negative or uninformative.
EY: I haven’t done [liquid biopsies] much. The big push-back for me is insurance. It’s a $5000 or more test. I’ve tried to do Guardant, and I may have this wrong, but they do not do their financial deal with a client until they get the specimen. I’ve gotten so much pushback from primary payers, at least in Portland. I think the key is to get adequate tissue, and the partnership between the person doing the procedure and the pathology department is enormous.
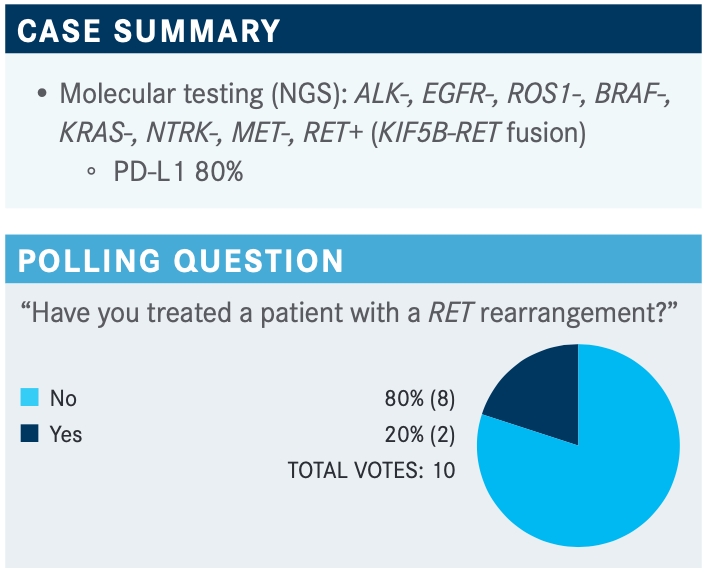
SANBORN: It looks like 80% have said no and a few people had said yes. That probably reflects the relative rarity of the RET rearrangement. For those who have treated patients with RET, how many patients have you treated?

CHEN: This was before the approval of selpercatinib [Retevmo]—a patient had a high PD-L1. He got immunotherapy up front and then later he progressed quickly. I referred him for a clinical trial of the RET inhibitor, and now it’s approved. He came off the clinical trial, and we have him on standard therapy now.
YANAGIHARA: In the past 3 or 4 months, I have treated 2 patients with RET fusions who I’ve put on pralsetinib [Gavreto]. One of the patients had de novo stage IV M1A because of pleural effusion, so he was started on pralsetinib as frontline therapy and did very well with no toxicity.
The other patient was a man heavily pretreated over several years. I looked through all his records and, in fact, he was RETrearranged. He’s gotten pralsetinib as a very late-line therapy, and he’s been on it for maybe 2 months or so.
CHEN: My patient did not have brain involvement at the time of diagnosis.
YANAGIHARA: My 2 patients with RET-rearranged NSCLC did not have brain involvement either.
SHAHIDI: I would send to radiation oncology to get radiation first and then systemic therapy.
KOSIEROWSKI: I don’t know if selpercatinib or pralsetinib crosses the blood-brain barrier to reliably cause regressions. In EGFR mutation, you rely on osimertinib prior to radiation therapy to see if you get a response first before proceeding, especially with multiple brain metastases. You don’t want to give whole-brain radiation if you don’t have to.
MITIN: In my practice, I try to push back on the radiation therapy for adaptations. I feel most medical oncologists want to have those patients radiated first, and I really try to convince medical oncologists to maybe repeat the brain MRI in 6 weeks if it is a very small lesion and see if it progresses on systemic therapy.
Even for stereotactic radiation therapy, typically, the smallest size of the target is 1 cm. Whether the lesion is 5 mm or 7 mm, we’re still treating that same area in the brain. I’m very happy to observe those patients with frequent brain MRI scans. At 6 weeks, a brain MRI scan is very common even if the patient had a couple of 5-mm lesions that are completely asymptomatic. I hope that the patient, especially with a history of no smoking, will have an EGFR mutation, and I have certainly had those patients, and I was very happy that I held back and I did not do radiation therapy.
A Yale study has shown a good response with immunotherapy for asymptomatic brain metastases. I hope immunotherapy will do the job, and I think it’s 20% to 30% response rates in these patients. If they are symptomatic or the lesions are big, then we must discuss the treatment. Observe patients with smaller asymptomatic lesions and see if the systemic therapy will do the job.

EY: I had to look at the National Comprehensive Cancer Network guidelines.3 I haven’t seen a patient with this mutation since it’s so relatively rare. I would go to the literature each time.
HARRIS: I would tend to go with the drug that had the first approval by the FDA for that indication because of more experience with it in a larger number of patients. But toxicity issues may govern what I pick.
CHEN: I think [ARROW] shows a nice response for patients who are either treatment naive or have had first-line therapy. The CNS response is also quite nice. I think these data are very promising. It seems like the adverse events [AEs] for the 2 drugs are similar in terms of the LFT [liver function test] abnormalities and hypertension.4
EY: The study confirmed that I would still take CNS metastases to the radiation oncologists because this study has only 8 cases of CNS metastases.4 I mean, these are highly selected people who end up on clinical trials. In the real world, people progress. They’re [nonadherent]. They don’t show up for their follow-up MRIs. That study confirmed my idea.
SANBORN: It’s certainly hard to draw conclusions when the numbers are in the single digits. That’s true.
EY: Anytime I can avoid a platinum doublet, I do. We’re getting more and more used to managing the various AEs, but there are all sorts of unknowns in terms of which drug is better and what the best sequence is.
I remember before ondansetron [Zofran], when there was no survival benefit to anything. I virtually never recommended chemotherapy for stage IV lung cancer except for small cell lung cancer.
KOSIEROWSKI: What do you do if you have advanced edema or uncontrolled hypertension? Do you reduce doses? And if you don’t get a response from the reduced doses, do you switch drugs?
SANBORN: There are some guidelines for management of hypertension, LFTs, or edema, but if you are at dose holds and dose reductions and you’re on medications that aren’t effective in getting you to where you want to be, I would say at least personally, I had to switch 1 patient to another RET inhibitor to try to change toxicities.
REFERENCES
1. Oxnard GR, Yang JC, Yu H, et al. TATTON: a multi-arm, phase Ib trial of osimer-tinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31(4):507-516. doi:10.1016/j.annonc.2020.01.013
2. FDA approves first targeted therapy for subset of non-small cell lung cancer. News release. FDA. May 21, 2021. Accessed September 10, 2021. https://bit.ly/3fCjEyT
3. NCCN. Clinical Practice Guidelines in Oncology. Non-small cell lung cancer, version 5.2021. Accessed September 10, 2021. https://bit.ly/3hxRKWD
4. Gainor JF, Curigliano G, Kim DW, et al. Registrational dataset from the phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET fusion+ non–small cell lung cancer (NSCLC). J Clin Oncol. 2020;38(suppl 15):9515. doi:10.1200/JCO.2020.38.15_suppl.9515
