Roundtable Discussion: Quinn Compares Chemotherapy Treatments in mCRPC
David I. Quinn, MD, MBBS, PhD and other oncologists, discussed disease management and the option of using an osteoclast-targeting agent for a 75-year-old man with metastatic castration-resistant prostate cancer.
David I. Quinn, MD, MBBS, PhD


David I. Quinn, MD, MBBS, PhD, associate professor of Medicine, director, Norris Cancer Hospital and Clinics, leader, Developmental Therapeutics Program at USC Norris Comprehensive Cancer Center, and the head of the Section of Genitourinary Medical Oncology, Division of Cancer Medicine and Blood Diseases, Department of Medicine at the Keck School of Medicine of USC in Los Angeles, CA, discusses a metastatic castration-resistant prostate cancer case with a group of peers during a Targeted Oncology™ Case-Based Roundtable event.
HERMEL: I would agree with an osteoclast-targeting agent because he does have bone disease. I think [his management is] reasonable in the setting of T2N0 disease with basically very high-risk disease.
QUINN: Anyone else disagree with that and would have done things differently?
EBRAHIMI: With that PSA [level] of 29.4 ng/mL, the bone scan being negative, and the right hip pain, I think I would have ordered an Axumin PET-CT scan at the beginning.
QUINN: I think we’re suspicious that that hip pain was not being picked up on the bone scan. No doubt they went and looked at it, but he may have had metastatic disease from the get-go. That’s a good point; we don’t know the other parameters—whether his alkaline phosphate level was up, whether he’s anemic.
What do you think about a PET prostate-specific membrane antigen [PSMA] scan as an alternative to PET Axumin?
EBRAHIMI: I’ve seen the data; they look pretty good. At least in my neck of the woods, it’s not available yet, but I think it’s a good tool to have.
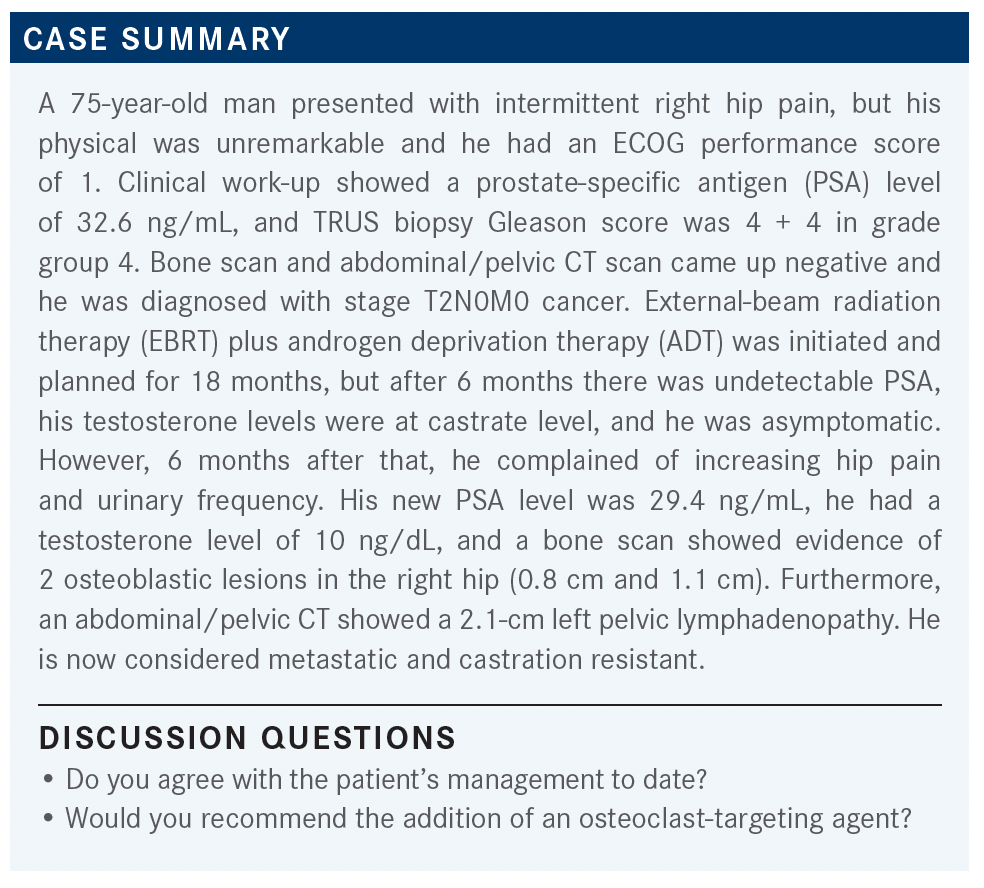
QUINN: Would anyone debate giving the patients an osteoclast-targeting agent at this point, and what would be your preference if you’re going to do that?
CHARU: I would consider giving him Xgeva [denosumab].
QUINN: What sort of regimen would you use at this point? Are you going to give it to him monthly or every 6 months?
CHARU: I’d probably start him on it monthly and then subsequently transition him later on.
QUINN: Do we have any Zometa [zoledronic acid] fans?
HERMEL: I would use Zometa. I think from an insurance standpoint, it’s very difficult, and it’s difficult to get the PET PSMA in the first line too. So doing non-Zometa as a first-line therapy is challenging.
QUINN: [The patient has] become castration resistant, so you might have a little more scope, and we know the PET Axumin was approved in the setting of a negative CT and bone scan or negative MRI and bone scan.1 So some of the insurers are resistant because of the cost, and when PSMA becomes more widely available, that may also be the case.
S. YEH: When you give Xgeva, do you give it every month, or can you space it out like Zometa? Because at Kaiser, where I work, we’re giving Zometa for 3 years and stop.
QUINN: There are 2 indications for giving these agents. Essentially, one is the maintenance or restoration of bone mineral density in the context of ADT. The regimens that are approved in hormone-sensitive disease are annual bisphosphonate, Zometa, and denosumab, given twice yearly, and that’s maintenance therapy. The approvals for these agents when we have bone metastasis are for when the patient is castration resistant, so let the insurers pick on that a bit. Then the regimen is basically a monthly regimen with each of the agents.
But in a practical sense, what we do at [the University of Southern California], is if the patient’s disease has responded, so that he does not have an active bone nodule—and the alkaline phosphate is controlled—and he’s responding to therapy, you can probably lean off a bit. This goes back to studies that were done when we first had Zometa, and then again with denosumab. What we do is we tend to give them bisphosphonate or their denosumab when they come for the monotherapy, typically every 3 months. Now, if they become active again, so they become castration resistant and they’re on leuprolide, goserelin [Zoladex], or whatever you want to use, then we tend to gear up again, and we’ll give them maybe 3 to 6 months of therapy but with monthly zoledronic acid or denosumab.
Now, is there an evidence base for that? We’ve been trying to develop [an evidence base] with the frequency splitting and do a study to compare, but we haven’t been able to do that because the revenue cycle of the drugs is relatively advanced. But we seem to do OK with that because you don’t want to overtreat, because of the risk of osteonecrosis of the jaw, which is small but there, with these agents.
MAR: For the maintenance indication, with the hormone-sensitive patients, do you do a DEXA [dual-energy x-ray absorptiometry] scan and then base your decision to start these drugs based on the T score, or do you just do it once you start them on the ADT?
QUINN: If you’re treating breast cancer, which I don’t do much of these days, you can get a DEXA scan at baseline. However, trying to get a DEXA scan for a guy going on hormone therapy with prostate cancer can be quite difficult, and they will approve it after a year. The problem is that our data suggest that you may have lost something by not having them on a bone health agent and calcium/vitamin D supplementation in that time. So usually, unless I suspect they’re osteoporotic for some other reason, and I get a hint from the CT scan, I don’t get a DEXA scan. I will check their vitamin D level, for example, if there’s a suspicion. I’ll do it at a year or at 2 years because I like to get some sort of baseline.
MAR: I get a DEXA scan, although you’re completely right that it’s a battle with insurance a lot of the time, I still start them. Once they start ADT, I still start them on the maintenance, but I feel as though DEXA, at least, can give me some guidance in terms of maybe backing off in the future, after a certain period with these drugs.
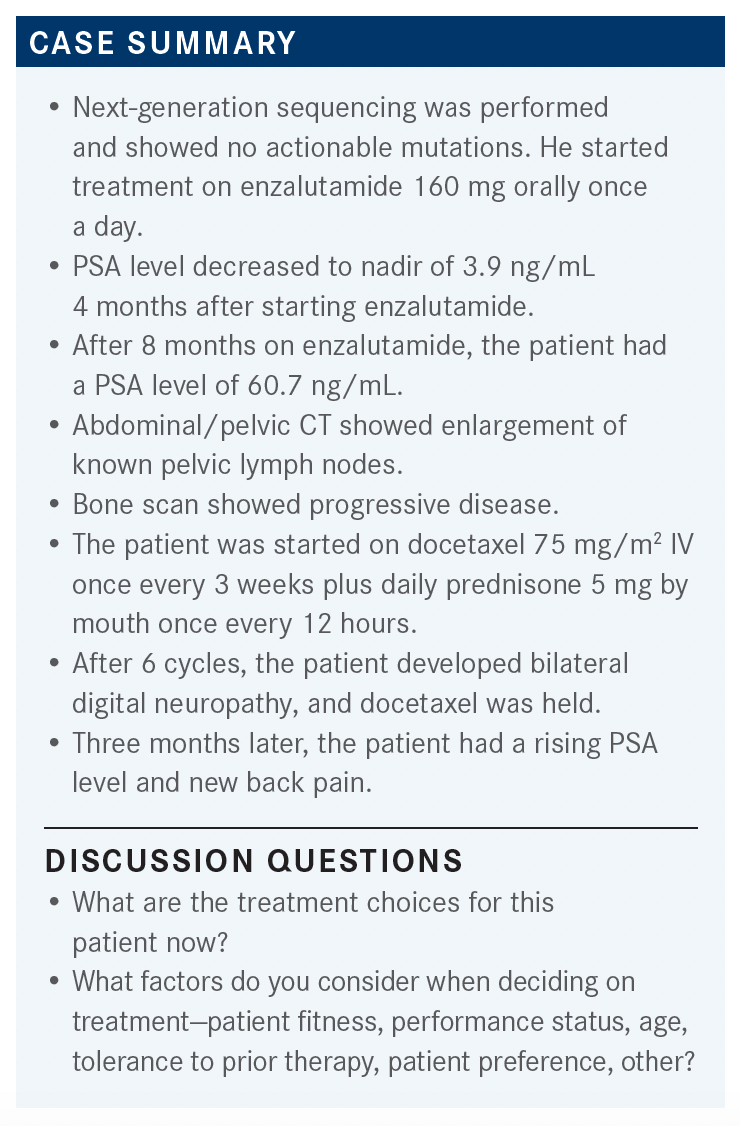
QUINN: How are you going to determine whether you’re going to give docetaxel or one of the oral agents?
S. YEH: In this patient, I picked docetaxel because he has a rapid recurrence of disease, bone metastasis within 6 months of his treatment, and 2 new bone metastases and is symptomatic and has [better lymph node involvement].
HERMEL: I would do chemotherapy if there was visceral involvement or more than 4 metastases, but in this case, it’s probably a preferable agent just for its tolerance.
CHARU: If you do chemotherapy, you can give the fixed duration and then continue them on ADT.
QUINN: How would you pick between abiraterone [Zytiga] and enzalutamide [Xtandi]?
H. YEH: I think, from a clinical trial, the rapid progression with the aggressive disease and early relapse—if they excluded highly symptomatic ones, and in terms of abiraterone studies and enzalutamide, that’s my understanding—I personally would not use the second-generation hormonal therapy in aggressive disease with a highly symptomatic population.
QUINN: I think that we have a limited evidence base. We’re not trying to design trials in the cooperative group that look at that bad actor group, from the hormone-sensitive group forward. I think there is a preference for us using chemotherapy there. Some people even do a taxane chemotherapy with carboplatin, [but overall we’re going with our gut on this]. Some people feel that the newer novel antiandrogens do as well as the chemotherapy, whereas others want to go with the chemotherapy first.
MAR: I think radium 223 [Xofigo] is not a bad choice at this point.
ALEXSON: I wouldn’t use any radium unless he’s having bone pain.
QUINN: Some people will pick an elevated alkaline phosphatase [level] as an indicator of potential benefit.
H. YEH: I think the cabazitaxel [Jevtana] has demonstrated survival benefit; based on the recent study, I think it’s reasonable in this setting. [The effectiveness in] the microsatellite instability–high population is extremely rare. I think it’s roughly only about 5%, right?
QUINN: It’s probably smaller than that, probably less than 2%.
H. YEH: That’s even worse, so the chance of even treating with immunotherapy is very small. I would think that chemotherapy at that stage, particularly cabazitaxel, demonstrates survival benefit. So it may be worthwhile considering [at this point].
CHARU: If you got abiraterone, you could go for enzalutamide because he was exposed to chemotherapy, so you can switch it and then try cabazitaxel later on. Or one can consider cabazitaxel up front.

CHANDURI: In general, I think, persuading patients [to undergo] chemotherapy in prostate cancer is not easy. In my practice, by the time they come, they’ll have poor performance status or some pain, and then usually with the chemotherapy, if they have some [adverse events (AEs)] already, it’s then very difficult to do more chemotherapy in this population.
ALEXSON: I think it depends on the marrow reserve. If he can tolerate the hematologic toxicity, I would give him cabazitaxel and see how he does. If he needs to reduce his dose, I will reduce his dose, but I think we would give him a try with it.
CHARU: I would consider cabazitaxel, too, maybe dose modified, and I feel that sometimes they tolerate it slightly better than the docetaxel in my practice.

JANG: For me, it’s just hard to believe the data. You have only 120 patients in each group. I’m a little surprised that AEs that lead to death are higher in the enzalutamide group than the abiraterone group. We all know that cabazitaxel, when it first came to the market, was marketed as a very toxic drug. It needed white cell boosters, severe myelosuppressions, and all that, but [it has since transformed] into a better drug than abiraterone and enzalutamide, which is, to me, kind of unbelievable.
QUINN: I was involved in reviewing the TROPIC study [NCT00417079], which was cabazitaxel vs mitoxantrone and a study that led to approval of cabazitaxel.3 There were several deaths [related to AEs on] cabazitaxel that occurred, but they all occurred in Eastern Europe on the study. There was a question about just what the supportive care was like. Growth factors were not used as a routine, so I hadn’t used cabazitaxel to that point, although I had done a liver impairment study. It’s not the same group of patients, and from that perspective I never really found it that toxic, but what was your experience when you used it?
JANG: It’s pretty myelosuppressive, and the study had already shown that, and I guess the first time it was on the market, it was 25 mg, which was the first approved dose. The study had shown 20 mg was just equal, and it’s noninferior to 25 mg. So, I think dose adjustment has a lot to do with the way we look at this drug from the beginning.
QUINN: Some other comparative studies demonstrated that there was less toxicity with the lower dose at 20 mg/m2. The outcomes for survival were similar, and the PSA response was higher with the higher dose. At this stage, we’re trying to minimize toxicity and treating for [overall survival], which I think is fine.
References
1. Songmen S, Nepal P, Olsavsky T, et al. Axumin positron emission tomography: novel agent for prostate cancer biochemical recurrence. J Clin Imaging Sci. 2019;9:49. doi: 10.25259/JCIS_139_2019
2. de Wit R, Kramer G, Eymard JC, et al. CARD: randomized, open-label study of cabazitaxel (CBZ) vs abiraterone (ABI) or enzalutamide (ENZ) in metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2019;30(suppl 5):v851-v934. doi:10.1093/annonc/mdz394
3. Oudard S. TROPIC: phase III trial of cabazitaxel for the treatment of metastatic castration-resistant prostate cancer. Future Oncol. 2011;7(4):497-506. doi:10.2217/fon.11.23

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More