Mo Discusses Transplant and Quadruplet Therapies in Transplant-Eligible Multiple Myeloma
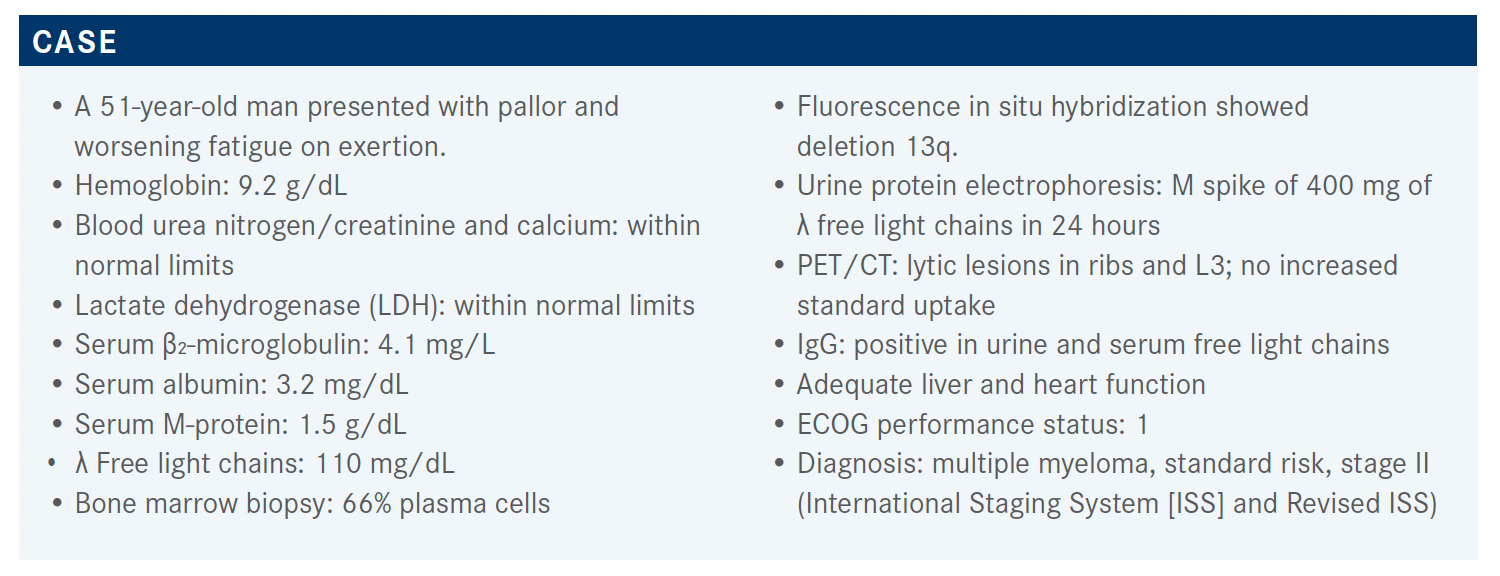
A 51-year-old patient who originally presented with pallor and worsening fatigue on exertion was diagnosed with standard-risk, stage II multiple myeloma.
Clifton C. Mo, MD

During a Targeted Oncology™ Case-Based Roundtable event, Clifton C. Mo, MD, assistant professor of Medicine, Harvard Medical School, director, Autologous Stem Cell Transplantation in the Jerome Lipper Multiple Myeloma Center for Multiple Myeloma at Dana-Farber Cancer Institute in Boston, MA, discussed the case of a 51-year-old man with stage II, standard risk multiple myeloma.
Targeted OncologyTM: What do the guidelines in this setting suggest for a patient such as this?
MO: The most recent NCCN [National Comprehensive Cancer Network] guidelines list the preferred and other regimens for patients with multiple myeloma who are considered [to be] transplant eligible.1 The preferred [category 1] regimen for multiple myeloma remains bortezomib [Velcade]/lenalidomide [Revlimid]/dexamethasone [VRd], [which, as the] NCCN category 1 [designation usually] implies, is supported by phase 3 data.
Cyclophosphamide/bortezomib/dexamethasone [CyBorD] is also an NCCN-preferred regimen, [whereas carfilzomib/lenalidomide/dexamethasone (KRd) and daratumumab [Darzalex] plus VRd are listed as other recommended regimens for multiple myeloma]. Ixazomib [Ninlaro]/lenalidomide/dexamethasone [is listed as] a category 2b regimen for multiple myeloma; [however], at least in my experience, this regimen is not commonly used. [A variety] of other combination regimens [are listed as useful in certain circumstances].
As a footnote, for all the daratumumab-containing regimens, the daratumumab and hyaluronidase [Darzalex Faspro] subcutaneous injection is considered an acceptable alternative to [the intravenous (IV)] formulation. The subcutaneous daratumumab injection has different dosing and administration instructions [than the IV formulation], with essentially a flat dose.
Which data do you think are valuable when considering early vs delayed transplant?
[It’s useful to review] relevant studies that may inform our decisions regarding the initial treatment regimen and strategy for multiple myeloma. The IFM/DFCI2009 study [NCT01191060] is a randomized study of VRd induction with or without early transplant, followed by a finite duration of lenalidomide maintenance.2,3 [In the study, all patients] received VRd for 3 cycles, followed by stem cell collection. Arm A went on to additional VRd for a total of 8 cycles, whereas arm B went directly to high-dose melphalan [plus] autologous stem cell transplant, followed by 2 additional cycles of VRd. Both study arms had 1 year of single-agent [maintenance with] low-dose lenalidomide followed by observation until progression.2,3 Thus, this was not a transplant vs no transplant study but an early vs delayed transplant study. The intent was for anyone in arm
A who relapsed to be consolidated in their second remission with autologous transplant if they had not received it in their first remission.
A North American counterpart to [the IFM/DFCI2009 trial], which was a European study, was led by Paul Richardson. [This sister study, called the DETERMINATION trial, was] designed to be similar, with 1 important difference: [IFM/DFCI2009] used 1 year of maintenance lenalidomide alone, [whereas DETERMINATION used] lenalidomide maintenance until progression or intolerable treatment-related adverse events [AEs].4
In terms of IFM/DFCI2009, the initial analysis was presented a little over 4 years ago at the American Society of Hematology [ASH] Annual Meeting in 2016 and published in the New England Journal of Medicine shortly thereafter.2 The analysis showed that the primary end point of progression-free survival [PFS] was met at a median duration of follow-up of about 4 years. [This translated to] a 14-month PFS advantage for early vs delayed autologous transplant [median PFS, 50 months vs 36 months]. The [PFS] benefit of early transplant was observed both in standard-risk and high-risk patients but perhaps with a hint of more benefit in the standard-risk [group].2
[Despite the] 14-month median PFS advantage in the early-transplant arm, at 4 years of median follow-up, overall survival [(OS) was similar in the early- and delayed-transplant arms (81% vs 82%)].2 An updated analysis presented at ASH 2020 revealed a PFS advantage of approximately 12 months in the early transplant arm.3 [However], very importantly, at a median follow-up of 8 years, the OS curves of the early- and delayed-transplant groups are still essentially superimposed [62% vs 60%].3
Also, the [curves for] PFS2—[that is, from initial randomization to a second progression]—were similar across study arms.3 Breaking [this] down further, the first PFS was [12 months] longer in the early-transplant [group], but the second PFS was about 11 months shorter in the early-transplant arm, so that the overall PFS2 curves [were similar across the study groups].3
[An analysis of the IFM/DFCI2009 data showed] that achieving minimum residual disease [MRD] negativity is prognostically good; among all trial patients, median PFS was longer in MRD-negative patients [not reached] compared with MRD-positive patients [29 months].5 [Further], this analysis showed that MRD negativity [had a greater impact on PFS] than the treatment arm. [Nevertheless], among MRD-negative patients, PFS was slightly longer among those who received early transplant vs those who received delayed transplant.5 [Similarly], MRD negativity is more important for PFS than baseline cytogenic risk. However, patients with high-risk disease had worse PFS in both the MRD-negative and MRD-positive groups.5
Can you discuss other important trials for the transplant setting?
Another important randomized study evaluating the effect of transplant [on outcomes in multiple myeloma] is the FORTE trial [NCT02203643].6,7 FORTE was similar to IFM/DFCI2009, [though it differed in its design in] some important ways. FORTE was initially a 3-arm trial in which patients were randomly [allocated] in a 1:1:1 ratio to the 3 study arms. In 2 of the study arms, patients received either KRd or [carfilzomib/cyclophosphamide/dexamethasone (KCd)] for 4 cycles, followed by early transplant, followed by an additional 4 cycles [of therapy with KRd or KCd, respectively]. In the third arm, patients received 12 straight cycles of KRd [KRd12] with stem cell mobilization and collection but without early transplant. A second randomization [was carried out] after the initial year of therapy, [which allocated patients in a 1:1 ratio to maintenance with] lenalidomide monotherapy vs carfilzomib/ lenalidomide dual therapy.
An updated analysis [of FORTE] presented at ASH 2020 showed that KRd plus early transplant had a statistically significant PFS advantage [over] KRd12 without transplant [78% vs 66%], as well as over KCd with transplant [78% vs 58%].7 The KRd12 arm had a higher PFS compared with the KCd transplant arm, but [this difference] was not statistically significant. A subgroup analysis revealed trends toward a benefit of KRd with early transplant [compared with KRd12] across major subgroups.
Analysis of data from the second randomization of FORTE demonstrated a significant PFS benefit [in favor of] maintenance with carfilzomib/lenalidomide dual therapy [compared with] lenalidomide monotherapy. Again, [this] trend was observed across major subgroups [defined by] stage, cytogenic risk, and [LDH level].7
How does quadruplet therapy compare with triplet therapy in multiple myeloma?
Another important phase 3 trial is CASSIOPEIA [NCT02541383], which assessed the value of adding daratumumab to bortezomib/thalidomide [Thalomid]/dexamethasone [VTd] in patients with transplant-eligible, newly diagnosed multiple myeloma.8 [Patients were randomly allocated in a 1:1 ratio to receive 4 pretransplant induction and 2 posttransplant consolidation cycles of VTd alone or VTd plus daratumumab (D-VTd)]; all patients received early transplant. The purpose of this study, [which was primarily conducted in Europe], was not to evaluate the benefit of early transplant but, rather, to assess the benefit of adding a CD38 monoclonal antibody to [a VTd] backbone. A second randomization was conducted to [evaluate maintenance with] daratumumab [for 8 weeks vs maintenance] until disease progression or up to 2 years.8,9
[At 100 days after transplantation, patients in the D-VTd group were] significantly more likely to achieve [the primary end point], stringent complete response [sCR], compared with those in the VTd group [29% vs 20%].8 D-VTd was also associated with a significant PFS advantage over VTd, equating to 53% reduction in risk of progression [or death at a median follow-up of 33 months (HR, 0.47)].8 Compared with the VTd arm, the D-VTd arm had about a 20% higher rate of MRD negativity as assessed by next-generation sequencing [57% vs 37%] or next-generation flow [64% vs 44%].8
What additional trials have looked at quadruplet regimens?
Another study, which is more directly applicable to the United States, is the phase 2 GRIFFIN trial [NCT02874742], which evaluated VRd vs daratumumab plus VRd [D-VRd].10 [In this study, patients were randomly allocated in a 1:1 ratio to receive 4 pretransplant induction and 2 posttransplant consolidation cycles of VRd alone or D-VRd, followed by dual DR [daratumumab/ lenalidomide] or R [lenalidomide] monotherapy for maintenance. Thus, as with CASSIOPEIA, in GRIFFIN, all patients received early transplant.] The primary end point of GRIFFIN was sCR; secondary end points were MRD, CR, and the overall response rate. The autologous transplant rate in the VRd arm [was 75% compared with 90%] in the D-VRd arm due to the early discontinuations.10
GRIFFIN met its primary end point, [with findings demonstrating a significantly] higher sCR rate in the quadruplet-therapy arm compared with the triplet-therapy arm [at a median follow-up of 26.7 months (63.6% vs 47.4%)].11 Further, the advantage of quadruplet vs triplet therapy was observed across multiple end points, including sCR, CR, and very good partial response [VGPR], and over multiple time points [ie, at the end of induction, transplant, consolidation, and 12 months of maintenance]. After 12 months of maintenance, over 80% [of patients who received quadruplet therapy had achieved] CR or sCR compared with 60% [of patients who received triplet therapy].11
Just as the depth of response [to quadruplet therapy] continued to improve over time [and] not just after transplant, so did MRD negativity. [In the overall intent-to-treat analysis], after 12 months of maintenance, MRD negativity was observed in 62.5% of patients in the quadruplet arm vs just over 27% of patients in the triplet arm. [Further], among patients who were specifically evaluable for MRD, quadruplet therapy was associated with nearly a doubling of MRD negativity compared with triplet therapy after 12 months of maintenance [MRD negativity rate, 78.3% vs 39.4%].11
In the subgroup analysis of GRIFFIN, odds ratios [for sCR and MRD negativity all] favored the quadruplet arm, [albeit] with some of the confidence intervals [crossing 1.00]—for example, in the cytogenetically high-risk sub group and the ISS stage 3 subgroup.11 [However], the numbers of patients in these subgroups were low, especially in the ISS stage 3 [group].
In terms of PFS and OS, overall, patients in the GRIFFIN trial did very well. At 24 months, PFS rates were 94.5% and 90.8% in the D-VRd and VRd groups, respectively.11 [Rates of] OS were over 90% in both arms and not significantly different,11 but the data [from this trial] are still relatively immature.
In terms of toxicity and AEs—specifically, hematologic AEs—[observations from the GRIFFIN trial] are probably what you would expect. Rates of AEs were fairly comparable [across the study arms], but higher rates of neutropenia, including grades 3 and 4 neutropenia, [occurred] in the D-VTd arm compared with the VTd arm.10,11 The D-VTd arm also had a slightly higher rate of low platelets and leukopenia [of grade 3 or 4 compared with the VTd arm],11 which is to be expected based on the known toxicity profile of daratumumab. The quadruplet therapy arm also had a higher rate of upper respiratory tract infections [compared with the triplet] arm; however, rates of infection of grade 3 or higher were similar, at just over 20%, in both study arms.10,11
In terms of nonhematologic AEs, both arms [of GRIFFIN experienced] fatigue, upper respiratory infection, and peripheral neuropathy at rates we would expect. Peripheral neuropathy and diarrhea were the most common nonhematologic AEs, which is expected, given the known toxicity profiles of bortezomib and lenalidomide. Rates of nonhematologic AEs of grade 3 or 4 were all less than 10%, and most were less than 5%.10,11 The rate of infusion reaction [was higher] with daratumumab-based therapy, as one would expect.
What do you think of these 4 trials you reviewed overall?
In summary, these 4 key studies, IFM/DFCI2009, FORTE, CASSIOPEIA, and GRIFFIN, have demonstrated much deeper responses and much higher response rates overall [to treatment of multiple myeloma] than we have seen in prior eras. [About 70% to 90%] of patients achieved at least a VGPR with any one of the treatment strategies [used in these trials]. The differences in design [of these studies] do not allow for direct, cross-trial comparisons; [nevertheless], impressive rates of PFS were associated with early transplant. Importantly, none of the studies demonstrated an OS advantage of early transplant vs [delayed] transplant [for patients with multiple myeloma]. High rates of deep response and MRD negativity were nevertheless associated with quadruplet therapies. Ongoing studies [in this area] include a phase 3 follow-up to GRIFFIN, called PERSEUS [NCT03710603], which evaluates D-VRd using [subcutaneous] daratumumab, as well as the GMMG HD7 trial [NCT03617731], which assesses a second anti-CD38 monoclonal antibody, isatuximab, in combination with VRd.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 1.2022. Accessed September 9, 2021. https://bit.ly/3fa9Yx5
2. Attal M, Lauwers-Cances V, Hulin C, et al; IFM 2009 Study. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311-1320. doi:10.1056/NEJMoa1611750
3. Perrot A, Lauwers-Cances V, Cazaubiel T, et al. Early versus late autologous stem cell transplant in newly diagnosed multiple myeloma: long-term follow-up analysis of the IFM 2009 Trial. Blood. 2020;136(suppl 1):39. doi:10.1182/blood-2020-134538
4. Randomized trial of lenalidomide, bortezomib, dexamethasone vs high-dose treatment With SCT in MM patients up to age 65 (DFCI 10-106). Clinicaltrials.gov. Updated February 18, 2021. Accessed September 9, 2021. https://bit.ly/2XhxaT6
5. Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456-2464. doi:10.1182/blood-2018-06-858613
6. Gay F, Cerrato C, Petrucci MT, et al. Efficacy of carfilzomib lenalidomide dexamethasone (KRd) with or without transplantation in newly diagnosed myeloma according to risk status: results from the FORTE trial. J Clin Oncol. 2019;37(suppl 15):8002. doi:10.1200/JCO.2019.37.15_suppl.8002
7. Gay F, Musto P, Scalabrini DR, et al. Survival analysis of newly diagnosed transplant-eligible multiple myeloma patients in the randomized Forte trial. Blood. 2020;136(suppl 1):35-37. doi:10.1182/blood-2020-136907
8. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29-38. doi:10.1016/S0140-6736(19)31240-1
9. A study to evaluate daratumumab in transplant eligible participants with previously untreated multiple myeloma (Cassiopeia). Clinicaltrials.gov. Updated December 7, 2020. Accessed September 9, 2021. https://bit.ly/3CbzNFh
10. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/ blood.2020005288
11. Kaufman JL, Laubach JP, Sborov D, et al. Daratumumab (DARA) plus lenalidomide, bortezomib, and dexamethasone (VRd) in patients with transplant-eligible newly diagnosed multiple myeloma (NDMM): updated analysis of Griffin after 12 months of maintenance therapy. Blood. 2020;136(suppl 1):45-46. doi:10.1182/ blood-2020-137109

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More