Novel Gene Panel May Predict Response to Tazemetostat in NHL
Tazemetostat showed efficacy in heavily treated patients with relapsed/refractory non-Hodgkin lymphoma in interim results from a phase II trial. Investigators hope that the analysis of a 62-gene panel biomarker performed on the same patient population will help to identify the patients who will have an even stronger response to the oral EZH2 inhibitor developed by Epizyme.
The open-label, multicenter, ongoing study was initiated with 5 monotherapy cohorts of 60 patients each, 3 consisting of patients with diffuse large B-cell lymphoma (DLBCL) and 2 consisting of patients with follicular lymphoma (FL). Patients were assigned to 800 mg of tazemetostat until progression, acceptable toxicity, or death. A total of 203 patients were included in the efficacy analysis and 210 in the safety analysis.
In results first presented at the 2017 International Conference on Malignant Lymphoma (ICML), the objective response rate (ORR) for patients with FL and EZH2 activating mutations (n = 13) was 92%. One patient (8%) had a complete response (CR), 11 (85%) had partial responses (PRs), and 1 (8%) had stable disease (SD). There was no incidence of pro- gressive disease (PD) in this group.1
Franck Morschhauser, who presented the results of the phase II study at ICML, noted that “molecular pro ling may help predict responses.”
The ORR was 26% for patients with EZH2 wild-type FL, including 3 CRs and 11 PRs (n = 54). In patients with DLBCL, the ORR was 29% for those with EZH2 mutations (n = 17) and 15% in patients without an EZH2 mutation (TABLE 1).
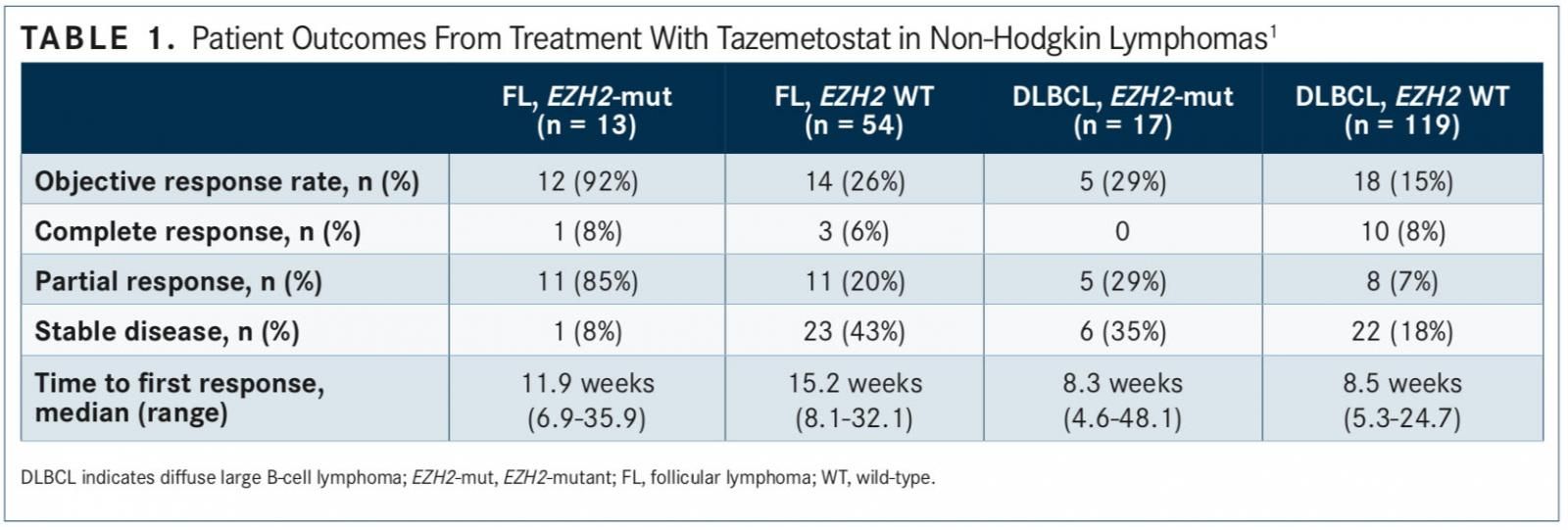
Eligible patients had undergone at least 2 prior treatment regimens. Most patients in the EZH2-mutant FL (46%), and FL wild-type cohorts (37%) had ≥5 prior regimens. The median number of prior treatments for both groups was 4. Hematopoietic stem cell transplant (HSCT) was noted in 23% of EZH2-mutant patients and 41% of wild-type patients in the FL cohorts. Patients in each cohort had a median Eastern Cooperative Oncology Group (ECOG) performance status of 0.
Most patients (41%) in the mutant DLBCL cohort had received 3 prior lines of therapy, while most patients (33%) in the DLBCL wild-type cohort had received 2 prior lines. The median number of prior treatments for both groups was 3. In the mutant cohort, 41% of patients had undergone prior HSCT and in the wild-type cohort, 24%. Patients in the DLBCL mutant and wild-type cohorts had a median ECOG performance status of 1.
The safety analysis showed that 90% of patients experienced an all-grade treatment-emergent adverse event (TEAE) and 59% had a grade ≥3 TEAE. Serious TEAEs were experienced by 39% of patients. The most common grade ≥3 TEAEs were thrombocytopenia (9%), anemia (8%), and neutropenia (7%).
Ten percent of patients experienced serious treat- ment-related AEs (TRAEs), and 15% had a TRAE that led to dose interruption. TRAEs led to dose reductions in 3% of patients and to discontinuation or withdrawal for 2% of patients. Serious TRAEs were observed in 10% of patients.
The most common grade ≥3 TRAEs were thrombo- cytopenia (6%), neutropenia (6%), and anemia (4%).
At the time of data cutoff, 48% of patients with FL and 12% with DLBCL remained on the study.
IDENTIFYING BIOMARKERS
Investigators led by Scott R. Daigle, principal scientist, Translational Medicine, Epizyme, conducted a 62-gene panel biomarker study among this patient population to identify for predictors for response associated with tazemetostat treatment.2
Daigle et al collected tumor and plasma-derived circulating tumor DNA (ctDNA) during screening and prospectively analyzed archived tumor samples (n = 203) for EZH2 gain-of-function (GOF) mutations Y646X, A682G, and A692V using the cobas EZH2 Mutation Test. Next-generation sequencing was performed retrospectively on DNA from archived tumor (n = 128) and pre-dose ctDNA (n = 185) samples to identify somatic mutations, applications, and translocations in a panel of genes that are commonly altered in NHL. Following the June 1, 2017, cutoff, patients were stratified into responders (CR + PR; n = 43) and nonresponders (SD + PD + unknown clinical response; n = 142).
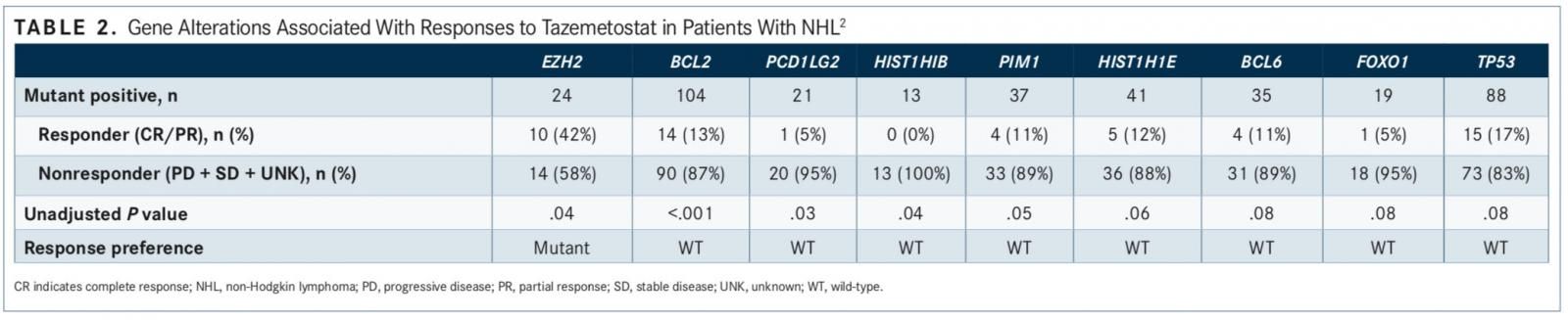
Analysis of all 185 samples, including patients with germinal center B-cell (GCB) DLBCL and FL, showed that mutations in EZH2 were associated with positive clinical responses (P = .04). Mutations in 8 genes were af liated with PD across the DLBCL and FL cohorts (all P ≤.08). Only BCL2 variants demonstrated signi cance through multiple test corrections (adjusted P = .05).
The majority of the tested genes had similar fre- quencies between cases of DLBCL and FL, except for PIM1 (28% and 6%, respectively), CREBBP (22% and 52%), and TNFRSF14 (10% and 29%). BCL2 and KMT2D were the most frequently altered genes in the patient population.
Overall, EZH2 (P = .04), STAT6 (P = .13), and MYD88 (P = .15) were associated with a favorable clinical response. HISTH1E (P = .06) and TP53 (P = .08) were associated with a lack of response (TABLE 2).
Analysis of 122 patients with DLBCL and 63 patients with FL identified genetic variants unique- ly associated with clinical response. In patients with FL, EZH2 GOF mutations were associated with a favorable clinical response (P = .002). Con- versely, genetic alterations of BCL2 (P = .06), BTG1
(P = .08), and KMT2D (P = .10) were associated with PD (FIGURE).3
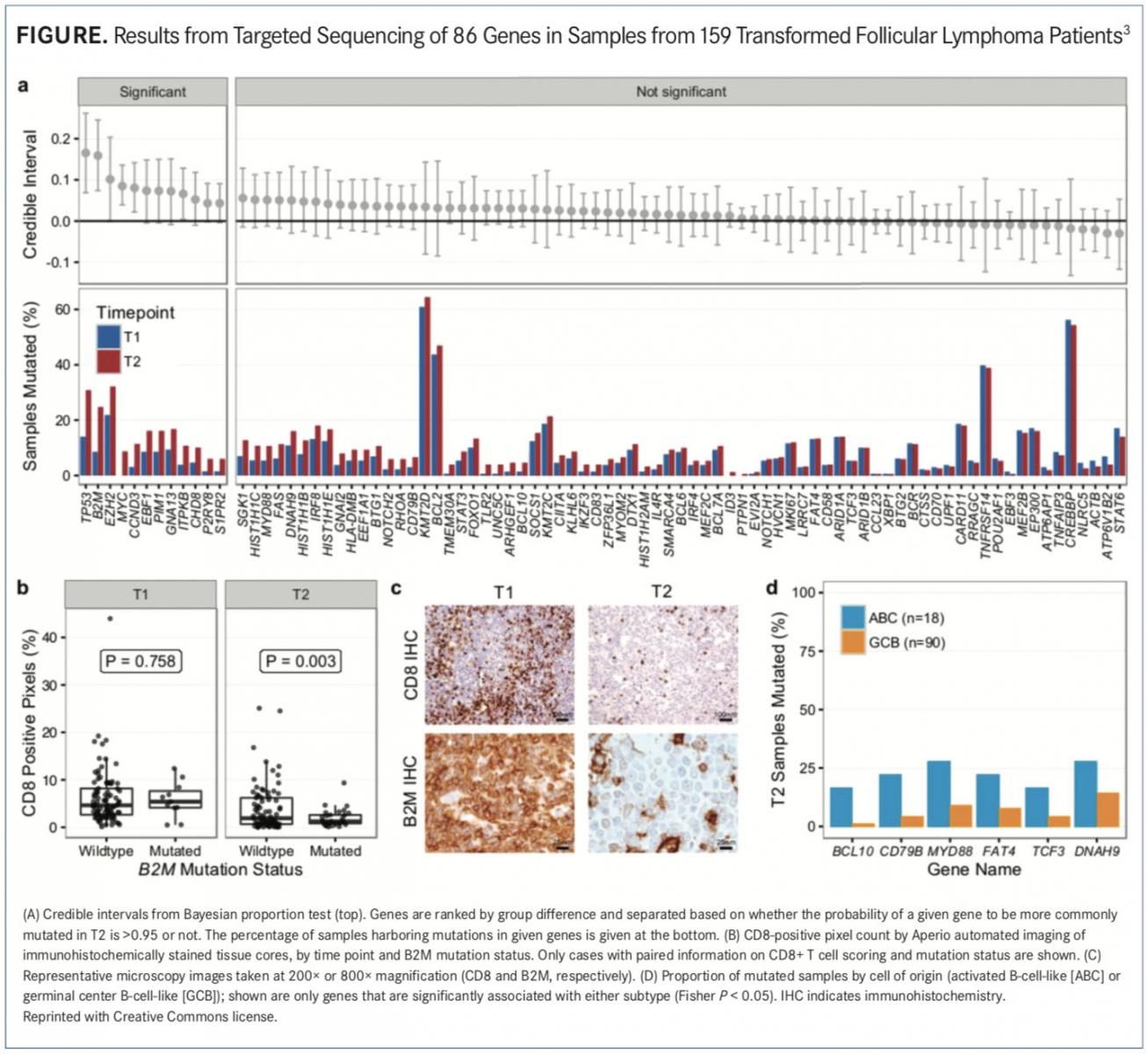
Investigators did not find any genes associated with a favorable clinical response in the DLBCL cohorts. Furthermore, alterations in BCL2 (P = .003) and CD274 (P = .07) were associated with PD.
Another research team lead by Stephen J. Blake- more, PhD, senior director for translational medicine, Epizyme, analyzed archive tumor- and plasma-derived ctDNA samples collected from 25 responders and 36 nonresponders at screening.4The samples were eval- uated using the same 62-gene panel used by Daigle et al.
As before, mutations in EZH2, STAT6, or MYD88 in patients with wild-type EZH2 were associated with response (P <.1), while mutations in HIST1H1E, TP53, or MYC (P <.08) were associated with nonresponse.
Patients matching a multigene predictor consisting of wild-type MYC and/or HIST1H1E but with mutated STAT6 and/or MYD88 in archived tumor samples had an ORR of roughly 58%. Patients who did not fit this profile had an ORR of approximately 19%, which researchers said is an indication that these 4 genes could predict response to tazemetostat.
“Molecular genetic pro ling of NHL patients identified potential predictors of response to tazemetostat beyond EZH2-activating mutations and offered new insights into mechanisms of response in EZH2 wild- type patients,” Blakemore et al wrote in their poster. “Plasma-based ctDNA screening may be a viable meth- od to identify patients [with NHL] with EZH2-activating mutations in the absence of archive tumor samples.”
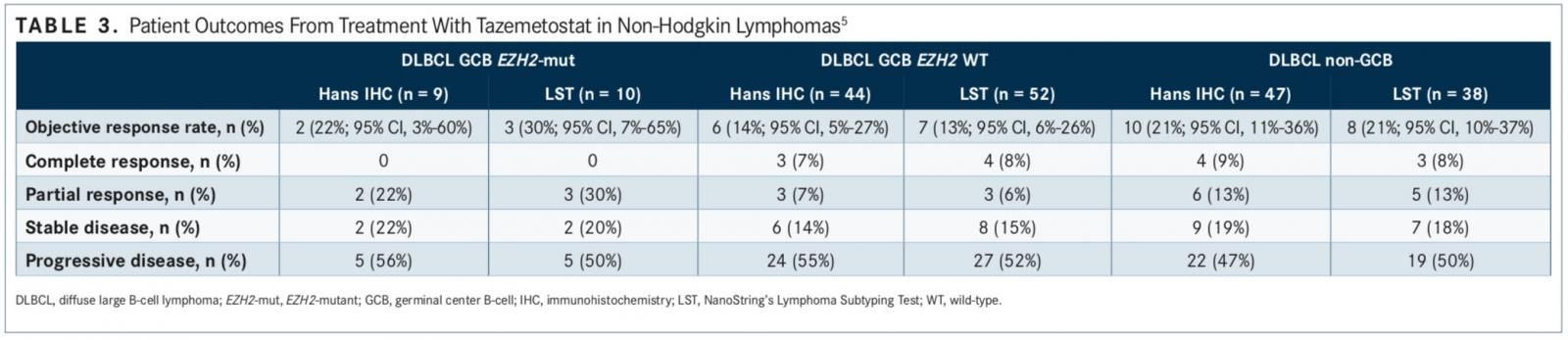
Finally, an analysis of patients with DLBCL (n = 137) presented at the 2017 American Society of Hematology Annual Meeting showed that there is not enough response data from patients with mutated EZH2 tumors to effectively assess the importance of cell of origin (COO). However, COO does not appear to be a primary driver of response to tazemetostat in patients with wild-type EZH2 tumors, leading investigators to believe that other factors independent of COO may be more predictive of tazemetostat activity (TABLE 3).5
References:
- Morschhauser F, Salles G, McKay P, et al. Interim report from a phase 2 multicenter study of tazemetostat, an ezh2 inhibitor: clinical activity and favor- able safety in patients with relapsed or refractory B-cell non-Hodgkin lympho- ma. Hematol Oncol. 2017;35(suppl S2; abstr NHL-255):24-25. doi: 10.1002/ hon.2437_3.
- Daigle SR, McDonald AA, Morschhauser F, et al. Discovery of candidate pre- dictors of response to tazemetostat in di use large B-cell lymphoma and follicu- lar lymphoma using NGS technology on ctDNA samples collected pre-treatment. Presented at: The International Conference on Malignant Lymphoma; June 14-17, 2017; Lugano, Switzerland. Poster 4013.
- Kridel R, Chan FC, Mottok A, et al. Histological transformation and progres- sion in follicular lymphoma: a clonal evolution study. PLoS Med. 2016;13(12). doi: 10.1371/journal.pmed.1002197.
- Blakemore SJ, Daigle SR, McDonald AA, et al. Preliminary evidence of a molec- ular predictor of tazemetostat response, beyond EZH2 mutation, in NHL patients via characterization of archive tumor and circulating tumor DNA. Hematol Oncol. 2017;35(suppl S2; abstr 154):159-160. doi: 10.1002/hon.2438_14.
- McDonald AA, Morschhauser F, Ribrag V, et al. Response to the EZH2 inhibitor tazemetostat is independent of cell of origin determined via Hans immunohisto- chemistry or Nanostring lymphoma subtyping test in EZH2 wild-type DLBCL pa- tients. Presented at: 2017 ASH Annual Meeting and Exposition; December 9-12, 2017; Atlanta, GA. Abstract 2745. ash.confex.com/ash/2017/webprogram/ Paper105158.html.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen