Despite Controversial 340B Payment Cuts, Study Suggests 85% of Hospitals Will See Payment Increases
As result of the Medicare Outpatient Prospective Payment System rule, 85% of 340B hospitals will see net payment increases in 2018, with rural hospitals reaping the largest benefits, according to an analysis conducted by Avalere Health that was commissioned by Community Oncology Alliance. This analysis compared 2017 Part B payments with 2018 payments and included 3814 hospitals.
Community Oncology Alliance
As a result of the Medicare Outpatient Prospective Payment System (OPPS) rule that took effect on January 1, 85% of 340B hospitals will see net payment increases in 2018, with rural hospitals reaping the largest benefits, according to an analysis conducted by Avalere Health that was commissioned by Community Oncology Alliance (COA).
Avalere analyzed 2016 Part B drug spending using the Medicare Standard Analytical File, which includes 100% of fee-for-service drug billing by hospital outpatient departments. To determine the total OPPS payment impact, the analysis compared 2017 Part B payments with 2018 payments and included 3814 hospitals covered under OPPS, not including 53 children’s and 11 free-standing cancer hospitals excluded from the rule.
“The Avalere study ‘2018 OPPS Medicare Part B Payment Impact Analysis’ is the first to investigate the net financial impact of a restructuring of payments for Part B drugs to correct the unintended consequence of some 340B hospitals profiting from the program,” COA said in a press release.
Enacted by Congress in 1992, OPPS necessitates that drug companies sell medicine to specified hospitals at a generous discount. With nearly half of all acute care hospitals participants of the program, many are culprits of hospital profiteering due to lack of transparency and accountability requirements. Prior to these changes, hospitals had access to upwards of 50% profits by selling drugs purchased at a discounted price through the program to insured patients.
Although many 340B hospitals predicted that the reductions in the program reimbursement would cause them a loss of revenue, research indicates that increases in Medicare Part B payments for non-drug items and services under OPPS have more than offset the 340B cuts.
Although, on average, all hospitals will see a 1.5% net payment increase, rural hospitals will see a greater benefit than urban hospitals, with a 2.7% net payment increase (TABLE). Further, research also shows that 42 states will see a net increase in total OPPS payments. The other states will see a decrease of, on average, 0.4%.
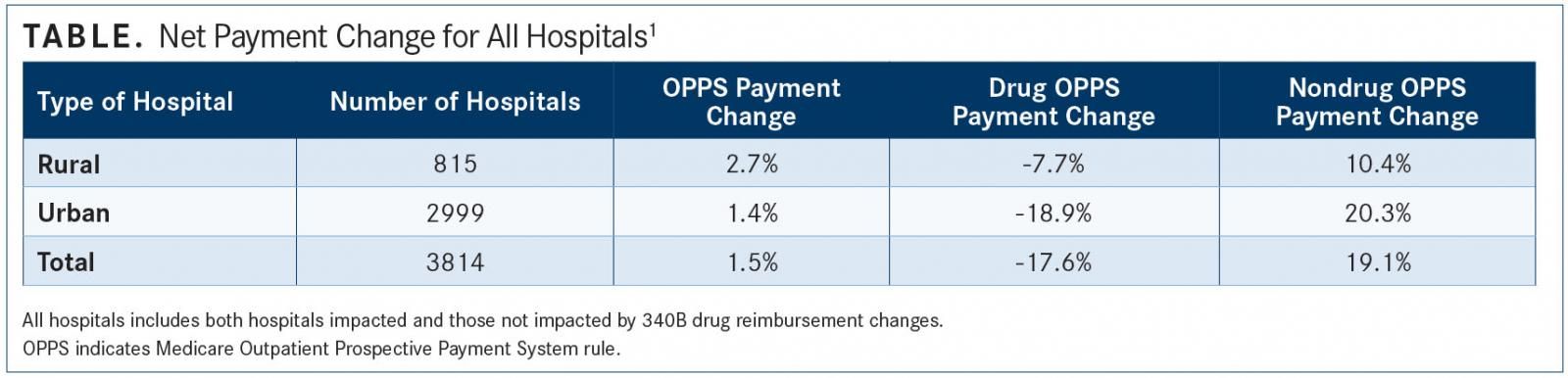
The analysis showed that rural hospitals will benefit most from the payment adjustments because 80% are exempt from the cuts. They will see greater than average overall net payment increases due to the reallocated savings, according to the analysis. For the hospitals not affected by the payment adjustments, they will see an average 4.4% net payment increase.
The final rule, announced in November, adjusted the payment for drugs purchased through the 340B program from the average sales price (ASP) plus 6% to the ASP minus 22.5%. The savings from the payment adjustments will be reallocated across all hospitals under OPPS.
Drug makers, who must agree to sell their drugs at a discounted price to covered entities in order to participate in Medicaid, have long encouraged more transparency and other changes to the 340B program. Nicole Longo, a spokeswoman for the Pharmaceutical Research and Manufacturers of America, accused the 340B program and “its perverse incentives” of distorting the entire healthcare marketplace, in a written statement.
While organizations like COA commended CMS on the reform, saying it is good for both patients and taxpayers and represents an important first step in stopping the abuse of the program by certain hospitals, other organizations denounced the rule. Ted Slafsky, president and CEO of 340B Health, said the limiting scope will benefit for-profit cancer clinics that turn away the poor, uninsured, and underinsured, and will not lower costs or expand access to care, particularly in rural communities.
America’s Essential Hospitals criticized Avalere’s study in a statement, claiming that the conclusions are skewed and “mask the real harm of the 340B reimbursement cuts.” The group also noted that the payment cuts “subvert the very intent of the 340B program by taking savings away from the safety net. For hospitals that operate on narrow margins, those that fill a safety-net role and depend most on 340B support, the payment cuts are unsustainable.”
Opposing views claim that Avalere’s study focused only on selective measures. While the study says that patient costs have been reduced with the CMS policy, Tom Nickels, executive vice president of the American Hospital Association, alleged in a statement that these cuts have actually increased copayments for 97% of Medicare patients treated at 340B hospitals.
The American Hospital Association, America’s Essential Hospitals, and the Association of American Medical Colleges sued HHS shortly after the rule was finalized last year, but a federal judge dismissed the attempt to block the cuts, saying that the suit was premature since the change has not yet gone into effect.
The payment change is among several legislations seeking to improve 340B program operations to help patients in need as opposed to hospital profits. These include the “Helping Ensure Low-Income Patients Have Access to Care and Treatment” and “340B Protecting Access for the Underserved and Safety-Net Entities Act” bills. COA claims to support both bills and is working with policymakers for more transparency and regulation to more effectively restrict program abuses.
References:
- 2018 OPPS Medicare Part B payment impact analysis. Community Oncology Alliance website. communityoncology.org/wp-content/uploads/ 2018/01/20180126_OPPS-Analysis-FINAL.pdf. Posted January 2018. Accessed January 30, 2018.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More