Khushalani Discusses the Use of Immunotherapy for Patients With Advanced Skin Cancer
The biopsy of a 88-year-man demonstrated infiltrative basal cell carcinoma. his case was the topic of discussion during a Case-Based Roundtable led by Nikhil I. Khushalani, MD.
Nikhil I. Khushalani, MD

During a Targeted OncologyTM Case-Based Roundtable event, Nikhil I. Khushalani, MD, the vice chair and senior member in the Department of Cutaneous Oncology at H. Lee Moffitt Cancer Center & Research Institute in Tampa, FL, discussed the case of a 88-year-old man with Basel cell carcinoma.

Targeted Oncology™: What are the typical treatment options for infiltrative basal cell carcinoma (BCC)? What stratifications are used to differentiate low-risk from high-risk BCC?
KHUSHALANI: The National Comprehensive Cancer Network [NCCN] guidelines list some of the anatomic [and] clinical characteristics of high-risk BCC. This patient has a tumor with ill-defined borders within the mask area of the face adjacent to the nose, [which is considered high-risk]. Other high-risk anatomic areas are [genitalia, hands, and feet].1
It is not highlighted whether this was a primary tumor or a recurrent tumor, but for the purposes of [this] discussion, we will assume it was a primary tumor. Certainly, it has an aggressive growth pattern primarily because of the infiltrating nature, extension, and irregular borders, so this would clearly classify at initial presentation as high-risk BCC.
Normally, when medical oncologists or dermatologists see patients within the early stages of their disease—particularly if they’re considered high-risk patients—they consider micrographic surgery or complete excision with circumferential and deep-margin excision. These would be the 2 primary types of surgical excisions undertaken for these high-risk patients.
What treatment would you consider for this patient?
In situations like this, we typically consider systemic therapy. The NCCN guidelines [lists principles of] systemic therapy for locally advanced BCC [laBCC] or metastatic BCC [mBCC].1 As a practicing cutaneous medical oncologist, the incidence of metastatic disease, including nodal metastases with BCC, or distant metastases most commonly to the lung or additional soft tissue are extraordinarily uncommon. These are probably in the incidence range of between 200 [and] 300 cases, or maybe even less conservatively, 200 to 400 cases per year in the United States.
The majority of patients who present with unresectable disease that requires systemic therapy are primarily what we define as those with laBCC for whom surgery would not be an option, primarily because of anatomic location, morbidity of surgery, or the disease developing in an area where surgery has [previously been undertaken, and in patients who fail after prior radiotherapy as well]. These are patients we certainly consider for systemic therapy. Hedgehog [pathway] inhibitors [HHIs], primarily vismodegib [Erivedge] and sonidegib [Odomzo], are both FDA approved in the United States for the management of laBCC, but only vismodegib is currently approved for metastatic disease.1
Immune checkpoint inhibitor [ICI] therapy with cemiplimab-rwlc [Libtayo], an anti–PD-1 agent, was approved by the FDA in February 2021 for patients who had laBCC or mBCC and who had either progressed on prior HHI therapy or [those who] were not candidates for HHIs.2
Many of you may have started using cemiplimab-rwlc in 2018 when it was originally approved for locally advanced or metastatic cutaneous squamous cell carcinoma [cSCC] based on the EMPOWER-CSCC-1 [NCT02760498] study data that demonstrated a response rate of approximately 48% for cSCC.3 Again, there is very little to choose across the anti–PD-1 pathway, but each one of them, including pembrolizumab [Keytruda], nivolumab [Opdivo], and the PD-L1 inhibitors, atezolizumab [Tecentriq] and avelumab [Bavencio], has very specific indications based on published trial data so far.
Cemiplimab-rwlc was the first immunotherapeutic agent that was approved for advanced BCC, but it is specifically for after failure of prior therapy with a HHI. It is important to recognize 1 unique characteristic in the eligibility criteria, [which] was if patients were on a HHI and failed to have an objective response after 9 months of therapy, which is typically the time one anticipates seeing these responses.1 These are patients in whom second-line ICI therapy can certainly be initiated. The [PTCH1 gene codes for the patched-1 (PTCH1)] transmembrane protein that typically serves as an inhibitor of smoothened homolog [SMO] in the normal physiologic state.4
How do gene mutations impact treatment decisions for patients with BCC?
[The] majority of sporadic BCCs, or the hereditary ones found with Gorlin syndrome, have inactivating mutations of PTCH1. The result is SMO escapes from the inhibition of PTCH, then causes downstream activation of glial cells, as well as subsequent activation of cyclin D1, which in turn leads to cellular proliferation and the development of tumors. Vismodegib and sonidegib inhibit the Hedgehog pathway, which is very important in embryogenesis. This is less important in adults because [the Hedgehog pathway] tends to be silent during that time frame.4 What’s critical to recognize—because this has a direct impact on adverse events [AEs]—is that this pathway is important for muscle restoration, particularly after traumatic or exercise-induced skeletal muscle injury.
But it’s also important in maintenance of normal homeostasis within hair follicles, in taste buds, and in normal muscles. Therefore, these are exactly the organ systems that tend to be affected from a toxicity standpoint and can sometimes be a bit difficult to manage.4 Vismodegib and sonidegib are both given daily [and] have relatively long half-lives, with vismodegib being approximately 4 days. Vismodegib can be given with or without food, but sonidegib needs to be [given] on an empty stomach. Certainly, both have a commonality in AEs of muscle spasms, arthralgias, alopecia, anorexia, nausea, weight loss, and potential increase in creatinine kinase, which can even lead to rhabdomyolysis [in extremely rare cases].5
The original ERIVANCE study [NCT00833417] that led to the approval of vismodegib was a small study published in the New England Journal of Medicine in 2012.6 The response rate [for vismodegib in laBCC] was 43% and slightly lower [at 30% for mBCC]. Sonidegib, which was in the BOLT trial [NCT01327053], had a very similar response for laBCC at about 44%.7

How long would you treat this patient?
A lot of the patients I see tend to come from rural, socially isolated backgrounds. Often when the BCC develops on the face, they tend to further isolate themselves, and by the time they get to us, it tends to have become disfiguring. Once we start them on therapy, over a period of 2 or 3 months, we can almost see a dramatic change in their psyche.
Please discuss the results from the ERIVANCE study.
There were 33 patients with mBCC in the original ERIVANCE study and 71 patients with laBCC, of whom 63 were evaluable for their responses. The primary end point was [overall response rate (ORR)] blinded by independent review, which was very critical because a lot of these were based on clinical evaluations and photographs. In this trial, photography of the lesions was critical, and the central reviewers painstakingly went through the pretreatment, then subsequently the treatment phase. Secondary end points were investigator-associated ORR, then progression-free survival [PFS], and finally, overall survival as well.6
The ORR in the original study was 30% for mBCC and 43% for laBCC, half of which were complete responses [CRs]. The median duration of response was approximately 8 months for these patients. A response of 30% or greater based on the RECIST criteria was defined as a response for these patients.6 There was a larger international trial called the STEVIE study [NCT01367665], which looked at over 1000 patients, and the ORR to vismodegib was about 69%. Similarly, the BOLT trial, with 230 patients, had an ORR of about 56%, so [there is] very little to use to determine choice between these 2 drugs. [It] eventually comes down to comfort and experience with the drug of choice.
What are some of the expected AEs? How do you manage toxicities? How do you counsel patients receiving an HHI?
Unfortunately, we don’t have lower-dose tablets for these patients, so alternate-day regimens have been tried. It’s 150 mg for vismodegib and 200 mg for sonidegib. Also staggering treatment by doing 3 weeks on, 1 week off, or 2 weeks on and 2 weeks off. There have been intermittent trials that have been conducted. For example, the MIKIE trial [NCT01815840] essentially had 3 months on and 2 months off. This was compared [with] a continuous duration of 6 months of vismodegib followed by a 2-months-on and 2-months-off dosing schedule. Essentially, there was equivalent efficacy and maybe a slight reduction in toxicity, so a variety of approaches have been tried.8,9
[However], a lot of the management ends up being symptomatic. For example, for muscle cramps, we advise patients to drink plenty of fluids and keep well hydrated. I’ve often told them to even try tonic water. Tonic water has quinine in it, and sometimes that has helped. Occasionally, we even escalate to a calcium channel blocker, such as low-dose amlodipine [Norvasc]. Amlodipine [given at] 2.5 mg to 5 mg, which sometimes is escalated to 10 mg, can also help mitigate against muscle cramps that tend to occur predominantly on the lower body.
Hair loss is a particularly distressing AE for patients. We’ve tried minoxidil [Rogaine] 5% topically. I’m not completely convinced that it really helps, but certainly the patient gets a sense that it works. The mechanism of toxicity for alopecia is fascinating and can sometimes be permanent. We have somewhere between 100,000 and 120,000 individual strands of hair on a full head of hair, of which 90% tend to be in the active growth phase, or the anagen phase, and about 10% are in the resting phase. These drugs often will prevent the resting phase to move into the active growth phase while destroying the active growth phase hair follicles as well. Unfortunately, the hair loss can be permanent. Less commonly, it can occur even after discontinuation of therapy for these patients.
The most common AEs are gastrointestinal, fatigue, weight loss, and decreased appetite.10 I have tried megestrol acetate [Megace] or even dronabinol [Marinol] with stimulation of appetite in these patients. Another troubling [AE] is dysgeusia. It’s very common for patients to tell us that food tastes terrible. Particularly, they seem to have an altered or vicarious sense of bittersweet taste, and a lot of their food tends to taste overtly salty, which makes them have an aversion to food and subsequent weight loss as well. I have seen improvement when I give them standard breaks from therapy.

What would you do next? What are your triggers to switch from an HHI? Would you stop therapy or switch to cemiplimab?
I would certainly image, particularly with either a CT of the maxillofacial area or an MRI of the face. Things we are unable to see on the skin, we can detect in the subcutaneous or muscle tissue. If there is any evidence of residual disease, definitive radiotherapy is very reasonable, reserving ICI therapy if he is developing future recurrence.

Would you ever biopsy to assess the response?
We’ve done that, but only in cases in which we were not quite sure. In this patient, who’s essentially completely epithelialized, I would not find a biopsy helpful because where are you going to biopsy? You can certainly perform random biopsies, and I almost guarantee these will be negative in a situation like this. I would much rather observe him if he had CR.
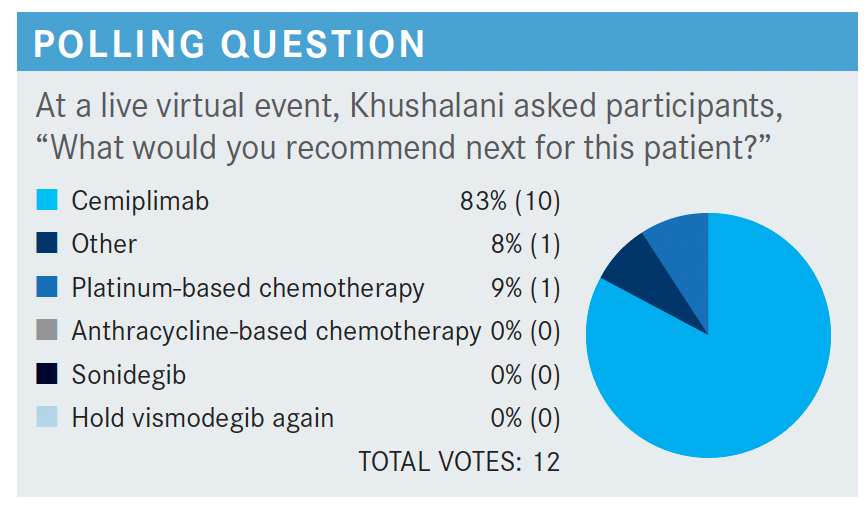
What would you have recommended next?
Given the approval of cemiplimab, I would probably wait for chemotherapy at a later point in a refractory patient and certainly can consider ICI therapy ahead of that, given the fact that BCC is UV light initiated. Therefore, [it will] often have a molecular signature that has some characteristics of UV damage signature, potentially higher number of neoantigens, therefore phonically a higher response rate because of systemic immunotherapy as well. The trial that led to the approval of cemiplimab [NCT03132636] has so far had peer-reviewed literature published for group 2, [which was made up of patients with laBCC].
The study stratified patients into those with mBCC, including both nodal and distant metastases, and those with laBCC. These were patients who had progressed on HHIs, had been intolerant to them, or had no better than stable disease after 9 months of therapy, so essentially, no objective response to HHI therapy. They received the FDA-approved dose for squamous cell carcinoma [SCC], which is 350 mg of cemiplimab intravenously every 3 weeks for up to 93 weeks. Tumor response, which was the primary end point in this trial, was measured by RECIST by an independent central review.11
The baseline demographic characteristics had patients that were predominantly in their seventh and eighth decades of life. Again, slightly more men, as is expected in this disease, which typically has a ratio of 1.6 men to 1 woman. Most of these patients had previously received vismodegib.
Interestingly, vismodegib plus sonidegib was also a regimen that was used. There’s no clinical or scientific rationale for using 2 HHIs simultaneously, or even switching from one to the other if a patient has developed disease progression, because the pathway interaction is the same.
The ORR was 31%, and 6% of patients had a CR to cemiplimab. The durable disease control, which was defined as disease control lasting for 6 months or longer, was almost 60% in these patients. Many of these patients had [early responses at approximately a median of 2 to 3 months], and because this occurred early, it would be easy to assess whether you’re going to continue therapy.
The PFS rate at 6 months was 76%, and it was 57% at 12 months. Even over time, patients who were not responding contributed to that median PFS dropping. This is not necessarily a cure, but many patients had durable responses with a median of 19 months.
Safety profile was very similar to that in patients with SCC. Fatigue, diarrhea, itching, pruritus, and asthenia were the most reported. Grade 3 or 4 AEs were distinctly uncommon in this trial, very similar to what we know of the safety profile of single-agent anti–PD-1 therapy.11,12
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Basal cell skin cancer, version 2.2021. Accessed October 15, 2021. https://bit.ly/37fg4Hn
2. FDA approves cemiplimab-rwlc for locally advanced and metastatic basal cell carcinoma. News release. FDA; February 9, 2021. Accessed October 15, 2021. https://bit.ly/2VjB5y8
3. FDA approves cemiplimab-rwlc for metastatic or locally advanced cutaneous squamous cell carcinoma. News release. FDA; September 28, 2019. Accessed October 15, 2021. https://bit.ly/3lM9TCJ
4. Low JA, de Sauvage FJ. Clinical experience with hedgehog pathway inhibitors. J Clin Oncol. 2010;28(36):5321-5326. doi:10.1200/JCO.2010.27.9943
5. Harris L. Basal cell carcinoma: a pharmacist’s guide. US Pharmacist. August 19, 2019. Accessed October 15, 2021. https://bit.ly/3xnc8Pd
6. Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171-2179. doi:10.1056/NEJMoa1113713
7. Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16(6):716-728. doi:10.1016/S1470-2045(15)70100-2
8. Villani A, Costa C, Fabbrocini G, Ruggiero A, Scalvenzi M. Dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor sonidegib to manage adverse effects: a retrospective case series. J Am Acad Dermatol. 2021;84(4):e211-e212. doi:10.1016/j. jaad.2020.12.006
9. Wong C, Poblete-Lopez C, Vidimos A. Comparison of daily dosing versus Monday through Friday dosing of vismodegib for locally advanced basal cell carcinoma and basal cell nevus syndrome: a retrospective case series. J Am Acad Dermatol. 2020;82(6):1539-1542. doi:10.1016/j.jaad.2020.02.050
10. Erivedge. Prescribing information. Genentech USA, Inc; 2020. Accessed October 15, 2021. https://bit.ly/3jlFDMw
11. Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):848-857. doi:10.1016/ S1470-2045(21)00126-1
12. Libtayo. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed July 25, 2021. https://bit.ly/3xlbjGH