Iyer Discusses Approved Therapy Combinations in T-Cell Lymphomas
During a Targeted Oncology Case-Based Peer Perspectives Roundtable, Swaminathan P. Iyer, MD, discussed FDA-approved therapy option for a patient with T-cell lymphoma.
During a Targeted Oncology Case-Based Peer Perspectives Roundtable, Swaminathan P. Iyer, MD, professor, Department of Lymphoma/Myeloma, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center discussed FDA-approved therapy option for a patient with T-cell lymphoma.
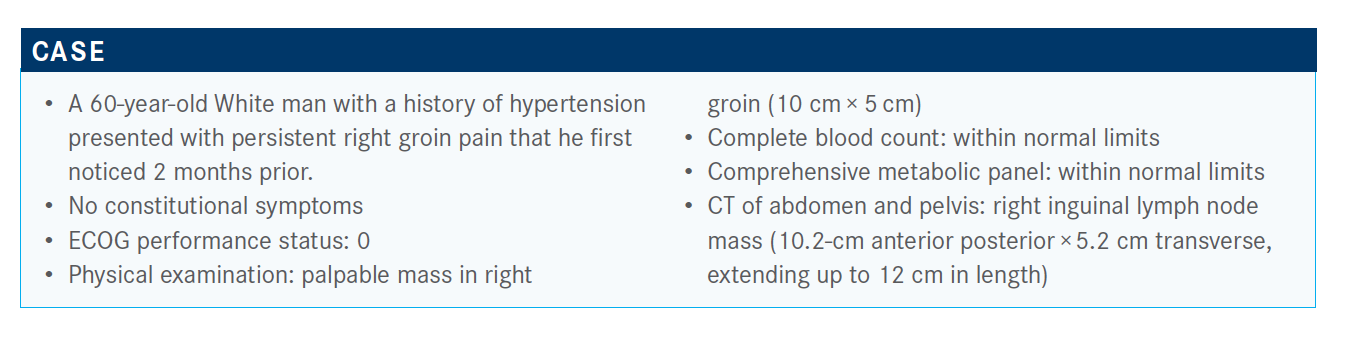
Targeted OncologyTM: What are your thoughts on these poll responses? Which approach would you choose?
IYER: The choice I would make [is] to do an excisional biopsy. The problem with a core needle biopsy in any lymphoma is the...cellular material required to make the diagnosis. Oftentimes, the cellular material [is limited] and you might end up with atypical lymph cells, which means you have to go back and do it again.
A multiple core needle biopsy helps [delineate] some of the [tissue] architecture. You want to stay away from FNA and single-core needles. I believe you would want to do anexcisional biopsy. Sometimes you can do a core needle, but then it’s followed up with an excisional biopsy.
What is your impression of the patient’s updated case?
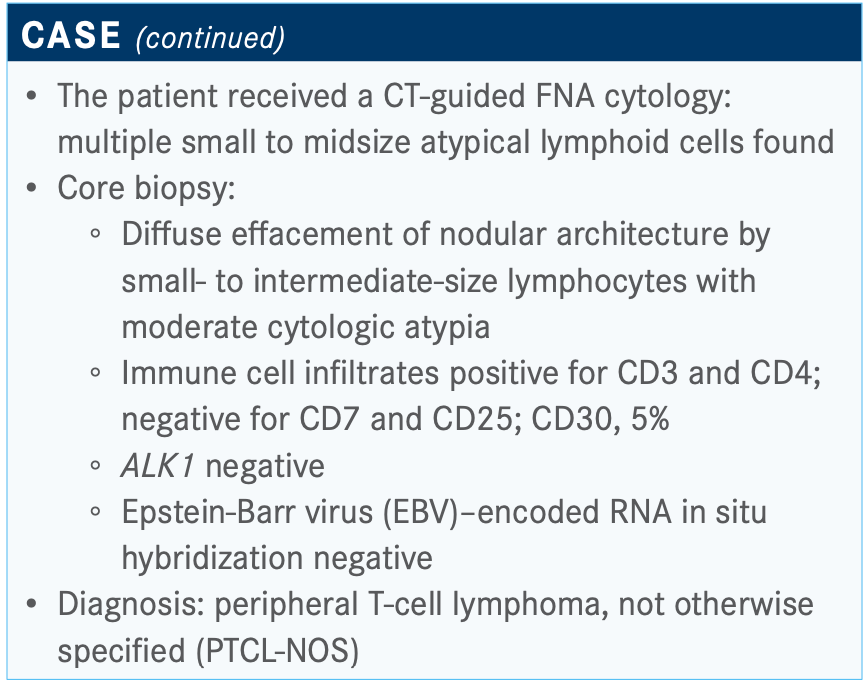
[The FNA] showed multiple small to midsize atypical lymphoid cells and that’s why you need to do a follow-up with a core biopsy. The core biopsy showed the architecture, which showed intermediate-sized cells with cytologic atypia and they’re all T cells—CD3 positive and CD4 positive.
The interesting thing about T-cell lymphoma is how do you determine the clonality, the loss of antigen? CD7 is the most common antigen that is lost in T-cell lymphoma. CD7 [in this patient was] lost; sometimes it’s CD7, as in this case, [or] it might be CD5. It might be CD2, but that’s rare.
The combination of CD3, CD4, and loss of CD7 gives you the clonal picture. The CD30 was 5%; that’s an important aspect. ALK1 was negative, and I would be suspicious of ALK1 positivity with a CD30 presently at 5%. EBV was negative, which is an important consideration. The diagnosis of PTCL-NOS was made.
Now, we are talking about PTCL-NOS, which was similar to diffuse large B-cell lymphoma, meaning everything was diffuse large B-cell lymphoma until we identified the cell of origin. Similarly, in PTCL-NOS, we now have a cell origin and now there are stains that can be done.
Which treatment would you most likely recommend for this patient?
Many times, we often end up using EPOCH [rituximab (Rituxan), etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin] as an extrapolation.
CHOEP [cyclophosphamide, doxorubicin, etoposide, vincris- tine, prednisone] is a popular one; [in other words,] adding etoposide to CHOP for T-cell lymphoma. A study many years ago looked at the benefit of etoposide, and it had a survival advantage in T-cell lymphomas. The choice is between EPOCH- or CHOEP-based approaches. I think CHOEP is perfectly fine. It’s outpatient friendly, community oncology friendly. I think CHOEP would be a good treatment consideration.
How do the National Comprehensive Cancer Network (NCCN) guidelines recommend treating patients in this setting?
In the NCCN guidelines...there is a longer list for first- line therapy [compared with Hodgkin lymphoma], and it is split into the subtypes of T-cell lymphomas.1 The preferred regimen is brentuximab vedotin [Adcetris] plus CHP [cyclophosphamide, doxorubicin, prednisone] for anaplastic large cell lymphoma [ALCL]. You can consider other regimens.
If you look at other histologies, that’s where the question [of treatment] is. That’s why this case scenario came up for discussion, whether it is PTCL-NOS, angioimmunoblastic T-cell lymphoma [AITL], enteropathy associated, monomorphic epitheliotropic intestinal T-cell lymphoma, nodal PTCL, or T follicular helper [TFH].
The pathologies are slowly changing. A big portion of that consists of the subgroup called the TFH phenotype....These T cells are often CD10 positive and BCL2 positive, and they have been carved out and put together in a category. They behave more like AITL.
In those situations, expression of CD30 can be anywhere from 30% to 60%. But if you look at expression itself, that’s variable. It could be 1%, it could be 5%. You can’t predict for that. That’s when you have a choice of regimen, brentuximab vedotin plus CHP, CHOEP, CHOP. Those are still done in many situations, and you have aggressive regimens, as well, which we don’t normally use.
I hope that something [physicians] would consider is to take [patients in the first line] to autologous transplant, because intensification is an important progression-free survival [PFS]–providing treatment.
Which trial looked at the regimen of brentuximab vedotin plus CHP that this patient received? What was the design of the trial?
ECHELON-2 [NCT01777152] patients were CD30 positive, [with] mature T-cell lymphomas—what used to be called peripheral T-cell lymphomas.2 The terms are interchangeable. Patients had mostly ALCL, and [they could be] ALK positive or negative. It was systemic ALCL, distinguished from cutaneous ALCL, which we often see in practice. Dermatologists may refer patients to [oncologists], and [these patients’ disease is] much more indolent. They often don’t require treatment. Most of the time, they respond to radiation...and require brentuximab. PTCL-NOS, AITL, adult T-cell leukemia/lymphoma, enteropathy- associated T-cell lymphoma, and hepatosplenic T-cell lymphoma were also included.
Patients had good ECOG performance status and no grade 2 or above [peripheral neuropathy]. The comparator was CHOP, and the [end points were] efficacy and safety data.
What are the data from ECHELON-2 for patients with PTCL-NOS?
This is one of the first, maybe the only study in frontline PTCL, which has the most positive data sets both in terms of PFS and overall survival [OS].
PFS was the primary end point. Brentuximab plus CHP was much better than CHOP. The median PFS with CHOP was 20.8 months, and the median with brentuximab plus CHP was more than double, 48.2 months, with a hazard ratio of 0.71 [95% CI, 0.54-0.93].
In the subgroup analysis, there was benefit across the board [with the study regimen]. But if you just focus on the disease, the problems with T-cell lymphoma is the heterogeneity of diagnosis. There was benefit in ALCL, ALK positive and ALK negative. There was definitely a benefit in PTCL- NOS. The hazard ratio was over the line at 1.00 [for] AITL... there’s a question of whether AITL benefits from this. But then the argument is that the numbers [were small]. There are only about 18 patients and 19 patients in [the AITL group and PTCL-NOS] cohorts, [respectively], which was a small subset of patients.
You need to have a larger data set to be able to come up with meaningful conclusions. Now there are ongoing studies [for these patients]. We have done a study that has accrued by adding etoposide to this combination with brentuximab maintenance with transplant. These are all non-ALCL patients.
Clearly there was a survival benefit for brentuximab plus CHP, so there’s not much controversy here at all in terms of the benefit that provides. It clearly beats CHOP, both in PFS and also in OS. There was a good OS hazard ratio with a protective effect of close to 40% [HR, 0.66; 95% CI, 0.46-0.95; P =.0244].
There were responses with brentuximab plus CHP at 83% versus 72% [with CHOP]. Complete response rates were 68% versus 56%.
The progressive disease [rate] was also only about 7% [in the study] arm as opposed to 14% with CHOP. But I would say it’s better—in most CHOP studies, the progression rates were close to 30% to 40% in the patients with T-cell lymphoma.
What do you think of these poll results? What would you use for patients such as this who progress in the front line?
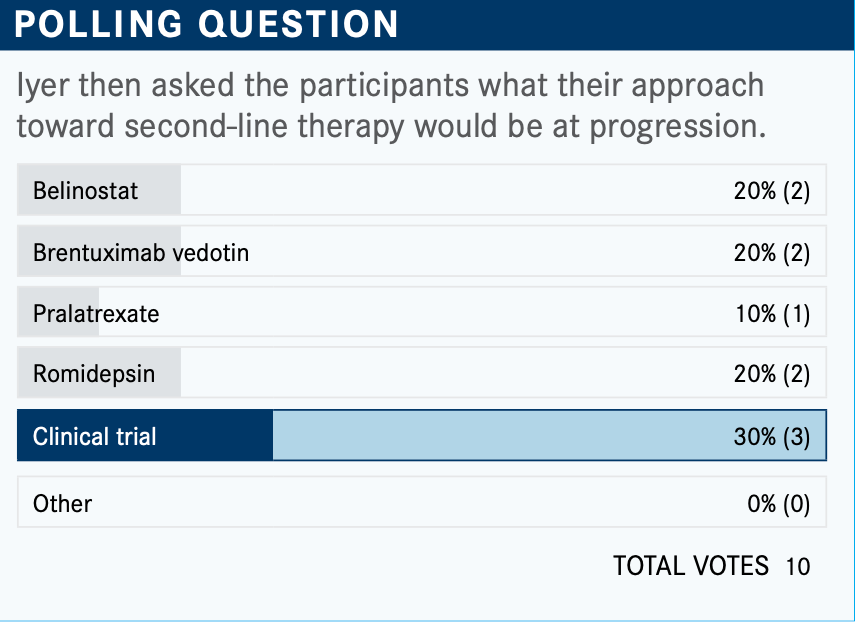
This one is a little difficult. The majority chose clinical study, which I think is a very good choice. There is a new CD30 [inhibitor] in addition to brentuximab that we’re looking at and that does not cause neuropathy.
There is the histone deacetylase inhibitor romidepsin [Istodax], which about 20% chose. Interestingly, many [feel] comfortable using belinostat [Beleodaq], and 20% would use brentuximab. Treatment with brentuximab is also an important consideration, particularly if it’s beyond 6 months to a year when relapse happens. [You] can always re-treat and get a good response.
The overall responses [with belinostat,3 pralatrexate (Folotyn),4 and romidepsin5 are] anywhere from 25% to 30%. The complete responses are 8% to 15%. The duration of response is probably the longest with romidepsin for more than 11 months. Survival data are only in those patients who achieved a complete response.
We have not made progress since 2014, when belinostat was approved, and 6 years later we have yet to have another drug that is approved for B-cell lymphoma.
[According to] the NCCN guidelines for relapsed/refractory disease in the second line, invariably you would end up using these agents.1 There is also the chemotherapy like DHAP [dexamethasone, cisplatin, cytarabine] and ESHAP [etoposide, methylprednisolone, cytarabine, cisplatin].
I think many [physicians] use gemcitabine, dexamethasone, cisplatin, or gemcitabine and oxaliplatin. Gemcitabine-based regimens are still a good consideration for your patients, [though] I would say these patients are few and far between. Trying to send patients to a clinical study would be ideal. Some of these studies—for example, the study that we are doing—will allow you to do standard-of-care regimens, and we can do the additional therapeutic [regimens] that go along without FDA-approved agents. I would say to consider those things.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. T-cell lymphomas, version 1.2021. Accessed December 16, 2020. https://bit.ly/2G0YfBi
2. Horwitz S, O’Connor OA, Pro B, et al; ECHELON-2 Study Group. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-240. doi:10.1016/S0140-6736(18)32984-2
3. Lee HZ, Kwitkowski VE, Del Valle PL, et al. FDA approval: belinostat for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Clin Cancer Res. 2015;21(12):2666-2670. doi:10.1158/1078-0432.CCR-14-3119
4. Parker T, Barbarotta L, Foss F. Pralatrexate: treatment of T-cell non-Hodgkin’s lymphoma. Future Oncol. 2013;9(1):21-29. doi:10.2217/fon.12.168
5. Barbarotta L, Hurley K. Romidepsin for the treatment of peripheral T-cell lymphoma. J Adv Pract Oncol. 2015;6(1):22-36.

Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More









