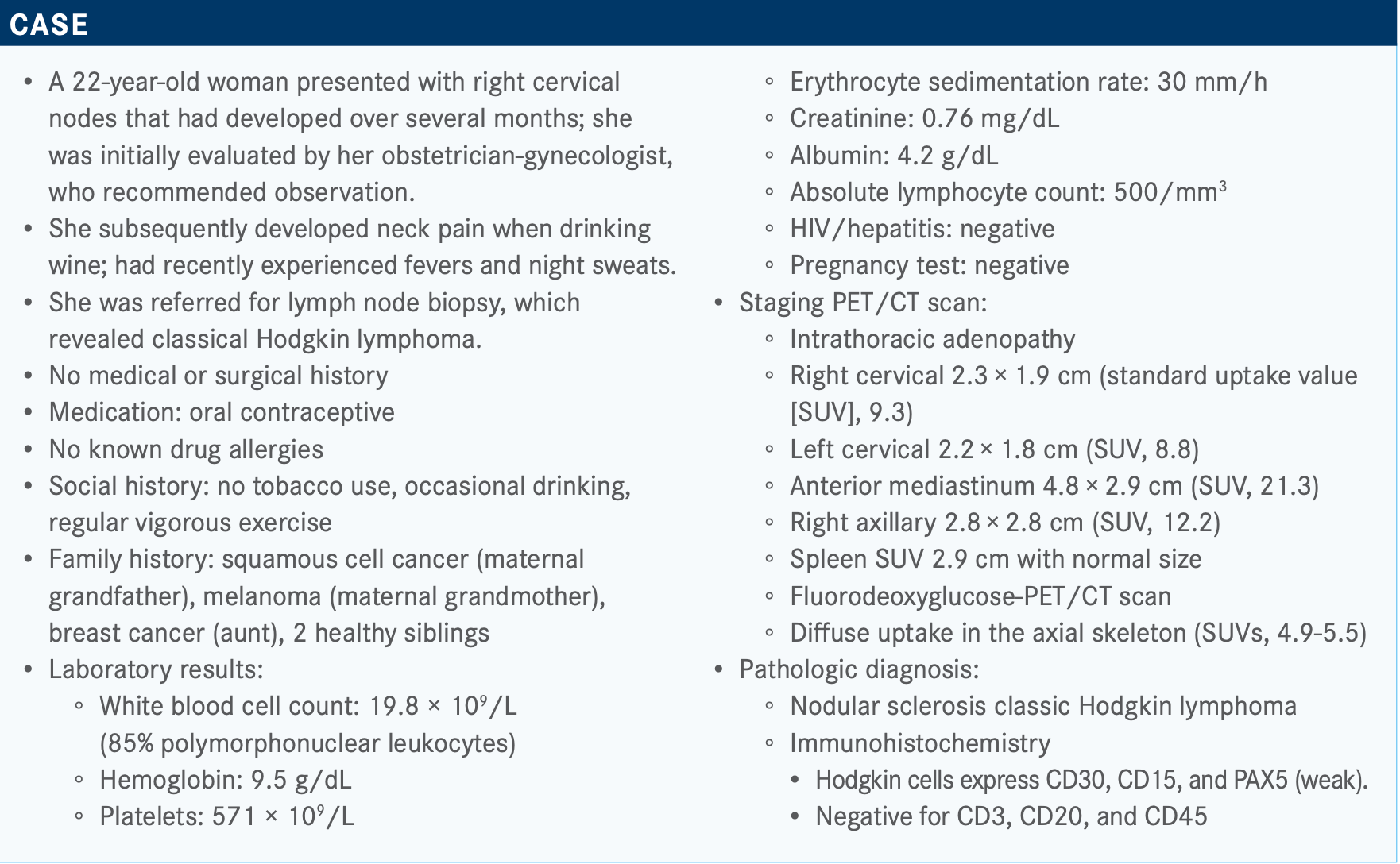
Expert Explains Why He Uses BV Plus AVD Instead of BEACOPP in Hodgkin Lymphoma
During a Targeted Oncology Case-Based Roundtable, Andrew M. Evens, DO, MSc, discussed testing and treatment for a 22-year-old patient with Hodgkin lymphoma.

During a Targeted Oncology Case-Based Roundtable, Andrew M. Evens, DO, MSc, an associate director of Clinical Services and director of the Lymphoma Program at Rutgers Cancer Institute of New Jersey, as well as the medical director of Oncology Service Line at RWJBarnabas Health in New Brunswick, NJ, discussed testing and treatment for a 22-year-old patient with Hodgkin lymphoma.
Targeted OncologyTM: Would you order additional testing for this patient at this time?
EVENS: We used to do bone marrow [biopsies], but now—at least for younger patients, if not for everyone—a bone marrow [biopsy] is not more sensitive [than a PET scan]. When you look at positive/predictive/negative, even though she had bone uptake, not bone marrow, you could argue you already know she’s stage IV. And so, we usually do not do bone marrow [biopsies], and at least [currently] there is no molecular testing [for Hodgkin lymphoma]. This isn’t like some of the solid tumors or even non-Hodgkin lymphoma. There’s no MYC or other molecular testing to do. We want to have more molecular markers. The only other test you would think about— [and] you don’t have to get it if you’re going to give bleomycin— might be pulmonary function test. We still get echocardiograms, not because we expect there to be any problems in a healthy 22-year-old, but we just want a baseline. We’re not going to repeat it. We just want a baseline for future reference [before we give] anthracycline....[But there is] no other real testing at this point.
Do you calculate prognostic scores for your patients with Hodgkin lymphoma?
I don’t vary treatment based on [a prognostic score]....It’s prognostic, it doesn’t tell me what to do different, per se. I know NCCN [National Comprehensive Cancer Network] guidelines prompt us one way, but...it’s not critically wrong if you don’t.
There are 2 issues with [the International Prognostic Score (IPS)].1 One, [it was] published in 1998. Two, there’s no real diving down score. Yes, there is some...updated literature where a lot of these [survival] curves have come together. If you use ABVD [doxorubicin (Adriamycin), bleomycin, vinblastine, dacarbazine] and other modern therapy—for what it’s worth, this patient would qualify with higher-risk disease by the IPS score [because of her] anemia, stage IV [disease], [white blood cell] count, and lymphocyte count being low.
What are your thoughts on the results of the poll? What would you have chosen of these options?
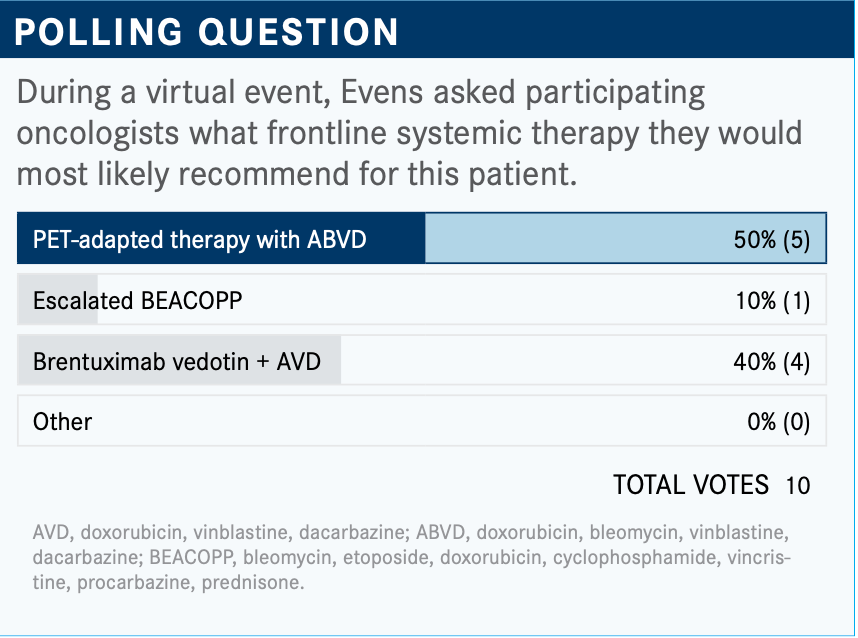
OK, so [half of the respondents chose] PET-adapted therapy with ABVD, so-called RATHL [response-adapted therapy for advanced Hodgkin lymphoma]; some would give her escalated BEACOPP [bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone], hit her hard out of the gates. Others [chose the] ECHELON-1 [NCT01712490] [regimen], brentuximab vedotin [BV; Adcetris].
I would have chosen BV plus AVD [doxorubicin, vinblastine, dacarbazine] for this. I used to give RATHL all the time. I’ve become dissuaded from using BEACOPP. I had a patient [to whom] I’d probably given it 25 times, and I can tell you it’s 25 hard times. One patient developed leukemia. Another one had osteonecrosis, and she was in her 30s. It was a nightmare, what you can see with BEACOPP; it’s not a wrong therapy, though.
Before the ECHELON-1 trial, [I would have used BEACOPP more] because it was more gestalt. If anything, there’s a PFS [progression-free survival] advantage, but it’s a hard treatment, especially escalated BEACOPP. [To me, it’s] the hardest outpatient treatment to give. You have to give Aloxi [palonosetron hydrochloride]/Emend [aprepitant]. It’s a really tough therapy. By the way, do not give it to anyone over age 50 or 60. Even with baseline BEACOPP, the treatment-related mortality rate is [greater than] 20% for patients over age 60. If you had a patient such as this who presented 6 years ago, and we knew her outcome with ABVD was 60, we would say, “Oh, let’s do something.” Now that we have another alternative that’s better tolerated, frankly, and maybe as effective, [although] it’s never been tested head-to-head, I don’t have a reason to give it nowadays.
How do you decide between PET-adapted ABVD or non– PET-adapted therapy?
PET-adapted [would] let me start with ABVD, and if my PET-2 is positive, meaning if it shrinks but I have a site still as hot as the liver, I’m then going to change to escalated BEACOPP, which ends up being about 20% to 25% of patients. The problem is that you ask [practitioners if they escalate to BEACOPP] and most say, “You know what, no.” So...if you keep giving ABVD in a [patient who is] PET-2 positive, your 4-year PFS [rate] is about 45%. That’s another reason why I’ve morphed to [giving] BV plus AVD; you still get a PET scan, but you’re not changing or escalating.
Do you use radiation for patients with advanced-stage disease?
No. The only caveat might be if someone in a weird case came in with advanced stage and a 15-cm mass...and maybe it would consolidate. There are some data that bulky disease in advanced stage is a poor prognostic factor. We’re not sure what to do about it. The Germans use a PET scan. But my problem is if someone’s still PET positive, you do see 1% or 2% who are primary refractory.
What do you consider to be the most important factor when deciding on a treatment recommendation for someone such as this patient?
These are hard questions because it’s rarely 1 factor that drives decision-making. It’s usually a lot of different things. So, even though [I said I don’t use] prognostic score, you’d like to know it. Of course, [knowing] performance status—can I be aggressive? Does she have lung disease? Smoking history really doesn’t affect anything....She’s 22, so you’ll be aggressive; if she were 72, you’d be less aggressive.
Another [factor is] fertility. The problem is BEACOPP causes infertility in a 22-year-old and at least half of women. You usually don’t have time. [A 22-year-old woman may not] have a partner. You [could] bank ovarian tissue, but it’s harder to do. It’s even harder if they don’t have a partner to do in vitro. We try to do it sometimes. At least the data with BV plus AVD would tell us we don’t think it affects fertility. You can’t say 0. For ABVD, I’ll quote1% to 2%. But BEACOPP is a bigger issue with fertility.
What would the guidelines recommend for such a patient?
The NCCN guidelines [are] a little formulaic in Hodgkin lymphoma.2 My friends and colleagues give ECHELON-1 here a category 2A [recommendation]. Where I disagree with my esteemed colleagues is when they talk more about high IPS or bleomycin being contraindicated. Well, to me, bleomycin being contraindicated is subjective. I would say bleomycin is contraindicated in almost everyone if I don’t have to give it on that. I, personally, do not use IPS to select it. They still try to steer patients toward RATHL. The problem is many patients, I can tell you, do not end up going to escalated BEACOPP. But I, frankly, don’t blame them because it is a tough therapy.
What data support the use of this regimen?

This is the alpha [trial that] we’ve already mentioned several times—ECHELON-1, an international phase 3 randomized study.3 A lot of the response-adapted data had not matured yet, so that’s why this was not, in part, response adapted. The standard arm was ABVD. At the time, the experimental arm—well, it’s now approved—was BV plus AVD. BV given 1.2 mg/kg on the same days as AVD, days 1 and 15. Patients received 6 cycles. They did a PET-2 scan but did not recommend changing therapy based on this. All [patients were] advanced stage...and over age 18.
The 2-year [data were] published in the New England Journal of Medicine in 2018.3 David Straus, MD, gave the 3-year data [of 83.1% with BV plus AVD versus 76.0% with ABVD].4 Usually in the Hodgkin lymphoma setting, [almost] 99% of all data is about PFS. It’s going to be hard in Hodgkin to show overall survival [OS]. We know we can salvage patients with transplant and checkpoint [inhibitors], etc. What you’re left with is an absolute improvement of 7 percentage points. To some people, that’s modest. To others, [including] me, that’s pretty good. I think it’s hard to ask 1 drug in lymphoma, or any cancer, to go up that point. This is, to me, also where hazard ratio is helpful.
The hazard ratio [was] 0.7. The way to phrase that is that it was a 30% risk reduction in PFS, which is progression or death. There [are] 5-year data that were presented at [the American Society of Hematology annual meeting] that basically show the same thing [of 84.7% with BV plus AVD versus 78.8% with ABVD in patients who are PET2 negative].5
There are relapses. Most relapses happen in the first 2 years, 80%. But 15% to 20% of relapses happen in years 2 to 5, probably closer to 15%. So, it was important to see this persistent threat and advantage at 3 years and 5 years.
Not surprisingly, on the forest plot pretty much everything was shifted over, favoring BV plus AVD. [Age] is [another underappreciated part] of all the BEACOPP data. I alluded to this with RATHL; nobody is over age 60. Anyone over age 60 is going to bring down [the Kaplan-Meier] curves. There were almost 200 patients on this ECHELON-1 study that were over age 60. Because if you’re not using BEACOPP, you have no upper age limit. There was a benefit to [patients] over [age] 60. It is shifted a little bit, it’s a much smaller [percentage of patients, only] 186 patients, but certainly, it is not worse. And you avoid the bleomycin on this.
Looking at other factors, pretty much any risk factor is kind of shifted over to the left [favoring BV plus AVD].
Response rates were slightly better with BV plus AVD.
What about the safety data from the ECHELON-1 trial?
Of course, in Hodgkin it’s not all about PFS and other efficacy [points]; we want to know about adverse effects [AEs] Because we want to cure and mitigate, minimize AEs, especially anything post therapy.
Most of it was balanced.3 There were a couple of signals [which] were increased [in one arm] versus the other. The pulmonary [effects were] much higher on ABVD, and there were a couple fatalities from bleomycin on ABVD.
What about BV plus AVD? Peripheral neuropathy, diarrhea, and febrile neutropenia were a little higher.
What about neuropathy? This is important and not surprisingly so. BV has antitubulin [antibodies] in it. It’s an antibody-drug conjugate, but the drug is monomethyl auristatin E, or MMAE. [It has] a little antitubulin [antibodies in it]. Thankfully, most [neuropathy seen with BV] was low grade. Most either completely resolved or improved over time.
Should you routinely be giving growth factor prophylaxis with BV plus AVD?
The answer should be yes. This one is a right or wrong. And it’s OK [if you aren’t] because [the investigators] didn’t know it when they started the study or didn’t appreciate it—they knew there was going to be higher neutropenia, but about three-fourths of the way through this study, they noticed the febrile neutropenia rate was a little over 20%. Then they instituted, or highly recommended, growth factor prophylaxis and cut it down to 11%.
So, even for younger patients, you want to use growth factor out of the gates. I give Neulasta [pegfilgrastim]. It’s probably more than we need, but it’s just easier for patients to get on the day of their visit.
Almost every case I’ve seen, in 1 in 10 patients, [febrile neutropenia comes up during] cycle No. 1. Sometimes if I’m worried about someone, I might add on some antibacterial Levaquin [levofloxacin] or something such as that to try to help decrease that. You want to use growth factor throughout.
What is your approach toward treatment selection for patients with comorbidities?
The No. 1, 2, and 3 [top] risk factors for bleomycin [are] age. And from looking at a lot of data, it’s exponential by decade. Usually, you don’t see it until the 40s, then 50s, then 60s, then 70s. A lot of it is because bleomycin is renally excreted. I guess renal function is an issue, too. The problem is that over age 70, the rate is almost 30% and there is a mortality rate of 20%. So, to me, you cannot predict it. In a random older patient, I’ve looked at everything. I’ve looked at smoking history, pulmonary function tests, echoes; it’s difficult [to judge outcomes based on comorbidities].
And [in] some of the bleomycin events, [the] benefits do not outweigh the risks. For older patients, I don’t use [bleomycin], or [for] anyone with pulmonary comorbidities.
What is your approach toward toxicity monitoring and treatment on BV plus AVD?
It’s [all about] communication. As it starts affecting function—instrumental ADLs [activities of daily living] and CTCAE [Common Terminology Criteria for Adverse Events]—working on a computer, a calculator, or cooking, laundry, etc—or if it’s ever painful, that’s a time to intervene. You hope it doesn’t affect self-care; that’s grade 3. The self-care [is] bathing, dressing, feeding, things such as that. So, [be] proactive, whether [a patient is] on BV or on CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone] for that matter.
The only other thing I’ll do, too, is for older patients who tend to get dehydrated. I’ll bring them in weekly for fluids or do home fluids...try to be more proactive. And then also—and you can argue [for this] for any older [patient with] aggressive lymphoma— I’ll sometimes do a little prephase steroids. Because for that older patient who comes in with 18 comorbidities, it’s always the first cycle [where] you run into trouble. You give them a little pulse of 100 or 60 mg a day for 5 days, you’ll see their performance status bump up a point. I think we don’t talk enough about prephase.
Would you dose reduce if you saw a patient had a neuropathy issue?
It’s an absolute balance beam. You don’t want to start dose reducing and then compromise their treatability and cure. It is a fine line, for sure. But neuropathy, [according to] the clinical trial, if it hits a certain grade, especially grade 3, there was a dose reduction. One dose level down to 0.9 mg of BV. What I do is bring down the vinblastine. We might try Neurontin [gabapentin] or Lyrica [pregabalin]....It doesn’t always work. I had a patient who had acupuncture, even though I don’t recommend that during treatment, but it helped.
If I have to bring down [the dose], if I had to choose between BV and vinblastine, I think BV is the more effective between the 2 tubulins. I’ll maybe bring down the vinblastine a little bit, and I’ve rarely had troubles after doing that. Maybe I can think of 1 case where I also had to bring down the BV. For most younger patients, you stay ahead of this. You’re able to do that and whichever one you bring down, I probably wouldn’t bring down both at once, just bring down 1 at a time.
Looking ahead, what other regimens are in development for the treatment of patients such as this one?
I am the ECOG [primary investigator] for [the S1826 study (NCT03907488)], so I’m a little biased. But that’s why I have to have equipoise to give an IPS of 1 BV because we’re looking at IPS and they’re stratified on it, but the patient can be IPS 0, and if they still have stage III advanced-stage Hodgkin, they’re eligible for this study....It took about 18 months to design it before [coronavirus disease 2019].
It’s open in North America—United States and Canada. It’s open to ages 12 and above. There is no upper age limit because we don’t have BEACOPP. We had a heated debate about our standard arm. We went to the National Cancer Institute at the end of 2018. The BV data came out that January, so [the data were] about a year old. We knew RATHL. The reason we did not use PET [is that] as a group we said we’re not sure PET response adapted is the future in Hodgkin. Why? When we looked at our study, and if you looked at RATHL, the majority of relapses happened in patients who are PET-2 negative. In other words, the 5-year PFS rate was right around 75% in PET-2 negative. So, even in PET-2 negative, a quarter of patients relapsed.
Now, for the small percentage who are PET-2 positive, it’s even worse. It was 65% lower. But when you look at the total numbers, we [asked ourselves], “If most of the patients who are relapsing are PET-2 negative, how helpful is PET-2 negative?” And we couldn’t do a 3-arm study, so we had to choose our standard arm for this advanced-stage study in North America. And long story short, we chose ECHELON-1 as our “standard therapy” for this.
[The experimental arm is] nivolumab [Opdivo] plus AVD. We’ve accrued about 250 of 1000 patients; there is still plenty of time. There are a bunch of correlatives [such as quality of life] that we’re going to be looking at, [including] metabolic tumor burden.
REFERENCES
1. Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on advanced Hodgkin’s disease. N Engl J Med. 1998;339(21):1506-1514. doi:10.1056/NEJM199811193392104
2.NCCN. Clinical Practice Guidelines in Oncology. Hodgkin lymphoma, version 1.2021. Accessed December 21, 2020. https://bit.ly/3oDAEZ4
3. Connors JM, Jurczak W, Straus DJ, et al; ECHELON-1 Study Group. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378(4):331-344. doi:10.1056/NEJMoa1708984
4. Straus DJ, Dlugosz-Danecka M, Alekseev S, et al. Brentuximab vedotin with chemo- therapy for stage III/IV classical Hodgkin lymphoma: 3-year update of the ECHELON-1 study. Blood. 2020;135(10):735-742. doi:10.1182/blood.2019003127
5. Straus DJ, Dlugosz-Danecka M, Alekseev S, et al. Brentuximab vedotin with chemotherapy for patients with previously untreated, stage III/IV classical Hodgkin lymphoma: 5-year update of the ECHELON-1 study. Blood. 2020;136(suppl 1):26-28. doi:10.1182/blood-2020-137089

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More