Evolving Paradigms in Lymphoma: Risk Stratification, Prognosis, and Current Treatment Strategies
This feature covers the "III. Risk Stratification and Prognosis" and the "IV. Current Treatment Strategies" sections of the current Evolving Paradigms in Lymphoma issue.
III. Risk Stratification and Prognosis
Hodgkin Lymphoma
Risk stratification varies somewhat by research group but generally divides the disease into early or advanced stages. The German Hodgkin Study Group (GHSG) and the European Organization for Research and Treatment of Cancer (EORTC) stratify early disease into favorable and unfavorable subtypes based on the presence or absence of risk factors. Unfavorable disease is usually defined by bulk and the presence of B symptoms. Conversely, the National Cancer Institute (NCI) and the National Cancer Research Institute (NCRI) do not subdivide early disease and classify patients with B symptoms and one or more risk factors as having advanced disease.4Clinical data suggest that the intensity of chemotherapy and radiation regimens can be reduced with early-stage disease, favoring identification of these patients as soon as possible.22
In advanced-stage HL, the international prognostic score, or Hasenclever score, is used to predict prognosis. The score is based on clinical parameters that include male sex, age >45 years, hemoglobin concentration <105 g/L, serum albumin concentration <40 g/L, stage IV disease, lymphopenia (0.6 x 109 lymphocytes/L or <8% of total white cell count), and leukocytosis (≥15 x 109 white cells/L). Each risk factor reduces the 5-year overall survival (OS) by approximately 8%.23The Hasenclever score should be used in conjunction with 18F-FDG PET imaging18for both early-stage and advanced-stage disease to assess risk. Overall, the prognosis for patients with HL is very good; the OS for patients with early-stage disease is often 90%; and the OS for patients with advanced-stage disease is 75% to 90%.24
Non-Hodgkin Lymphoma
The most widely used prognostic model for patients with NHL is the International Prognostic Index (IPI). Risk stratification (IPI score) is based on several clinical factors, including age >60 years, elevated lactate dehydrogenase concentration, Eastern Cooperative Oncology Group performance status ≥2, Ann Arbor stage III or IV disease, and ≥2 extranodal disease sites.25With one point assigned to each risk factor, patients are assigned to one of four risk groups: low risk, having zero or one clinical factor; low-intermediate risk, having two clinical features; high-intermediate risk, having three clinical features; and high risk, having four to five clinical features. Reported 5-year survivals of patients using this risk stratification are 73%, 51%, 43%, and 26%, respectively.25
The heterogeneity of NHL has proven challenging in finding a prognostic model that is effective for all subtypes, especially for those that are slow-growing, such as follicular lymphoma. In 2004, the Follicular Lymphoma International Prognostic Index (FLIPI) was developed to account for the differences in disease progression. The FLIPI shares three prognostic variables with IPI: age, Ann Arbor stage, and elevated lactate dehydrogenase. The FLIPI differs from IPI in two variables, specifically hemoglobin concentration (<12 g/L) and the number of involved nodal sites (>4).26Using these prognostic criteria, patients are stratified into low risk, having zero to one clinical feature; intermediate risk, having two clinical features; and high risk, having three or more clinical features. The 10-year survival rates for patients in these prognostic groups are 71%, 51%, and 36%, respectively.26
Though risk stratification using clinical factors is essential, gene expression profiling and other molecular techniques have emerged in recent years to further categorize NHL subgroups and provide prognostic value. One example of this advancement using genetic profiling has been in DLBCL. Profiling identified three additional subgroups: germinal-center B-cell-like, activated B-cell-like, and type 3 diffuse large-B-cell lymphoma. The germinal-center B-cell-like subgroup uniquely harbors 2 common molecular events, BCL-2 translocation and c-Rel amplification, and has the highest 5-year survival rate after chemotherapy among the subgroups.27Anaplastic large-cell lymphoma (ALCL) has also been divided into prognostic subgroups based on genetic profiling and the presence or absence of anaplastic lymphoma kinase (ALK) chromosomal rearrangements. The WHO has accepted three distinct subgroups: primary cutaneous ALCL, systemic ALK-positive ALCL, and systemic ALK-negative ALCL. Clinical outcomes vary with the subgroup; primary cutaneous ALCL is associated with excellent outcomes, ALK-positive ALCL is associated with favorable outcomes, while ALK-negative ALCL outcomes vary.28
Furthermore, PET scans are prognostic indicators of the response to initial therapy. In general, a negative PET scan after treatment signifies a better prognosis than a residual, positive PET scan.29The 5-point Deauville criteria are now the standard reporting method for treatment response.30
IV. Current Treatment Strategies
Treatment regimens for lymphomas are well established and have been included in the guidelines developed by the NCCN. Lymphomas are treated with combinations of chemotherapy, radiation therapy, and, more recently, with targeted therapy using monoclonal antibodies, antibody-drug conjugates (ADCs), and small molecule inhibitors. The remainder of this review will focus on these therapeutic strategies, with an emphasis on targeted agents approved in recent years and those still in clinical development. As more novel, potentially effective agents are being rapidly identified, clinicians should focus on incorporating these new agents into standard-of-care treatment regimens.
Current standard regimens in Hodgkin lymphoma
Classic HL treatment strategies are largely based on disease stage and number of risk factors. The most commonly used combination chemotherapy regimens, and those recommended by the NCCN, are ABVD (Adriamycin/doxorubicin, bleomycin, vinblastine, and dacarbazine), escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), and Stanford V (doxorubicin, vinblastine, mechlorethamine, etoposide, vincristine, bleomycin, and prednisone).10Patients with early favorable HL are commonly treated with two cycles of ABVD followed by involved-site radiation therapy (ISRT), while patients with early unfavorable HL can be treated with four cycles of ABVD followed by a slightly higher involved site radiation therapy (ISRT).22Escalated BEACOPP, considered a more aggressive chemotherapy regimen and used in unfavorable bulky disease, has shown some superiority in clinical outcomes, though not in OS, compared with ABVD.31
In recent years, therapeutic strategies have concentrated on reducing treatment-related toxicity while improving treatment efficacy for patients with early-stage disease. Clinical data have shown that decreasing the number of chemotherapy cycles and reducing radiation doses can be as effective as more aggressive regimens in patients with early-stage HL.22Recent studies have also evaluated radiation-free regimens with the hope of reducing treatment-related toxicity even more. PET scanning is crucial in the process of determining interim tumor response with this approach. In one study conducted by the NCRI, radiation-free treatment, using 3 cycles of ABVD and combined modality treatments, were found to have similar rates of progression-free survival (PFS) and OS at a 3-year follow-up. PFS was more than 90% for both treatment groups.32
For patients with advanced-stage (stages III-IV) HL, treatment cycles with ABVD or escalated BEACOPP are commonly used worldwide, although NCNN guidelines also recommend Stanford V in select cases. Clinical studies and a systemic review of the literature indicate significantly greater OS with BEACOPP therapy than with ABVD for patients with advanced disease.33-35Despite these data, ABVD continues to remain an acceptable frontline therapy because of the higher rates of acute toxicity associated with escalated BEACOPP. Nonetheless, a cure rate of 75% to 80% in patients with advanced HL is expected, independent of the first-line therapy chosen.3
Approximately 10% of patients with early-stage HL and 20% to 30% of patients with advanced-stage HL relapse after, or are refractory to, frontline initial treatment.4The clinical management of relapsed/refractory HL generally offers salvage chemotherapy followed by high-dose chemotherapy and autologous stem-cell transplant (ASCT). More than half of all patients with HL who receive ASCT will relapse. Second-line therapy for relapsed/refractory disease may include a number of multiagent therapies but typically include platinum-based or gemcitabine-based salvage regimens, such as ICE (ifosfamide, carboplatin, etoposide), C-MOPP (cyclophosphamide, vincristine, procarbazine, prednisone), or GCD (gemcitabine, carboplatin, dexamethasone).3Brentuximab vedotin (discussed in more detail below) can also be used as a second-line therapy if two prior combination chemotherapy regimens or ASCT have failed. Currently, brentuximab is the only targeted agent approved for the treatment of relapsed HL after ASCT.10
The prognosis for patients with relapsed/refractory disease depends on multiple factors, including stage at the time of relapse, time to relapse, and performance status. Patients who relapse within 3 months after initial treatment experience worse outcomes than patients with longer remission durations. Those who relapse within 1 year after ASCT only have a median survival time of 1.3 years after disease progression. As such, there remains a critical need to develop novel therapeutic agents that can improve outcomes for relapsed/refractory disease, as well as in the frontline setting.36
Targeted regimens in Hodgkin lymphoma
Although most patients with HL are cured with standard chemotherapeutic regimens, many experience shortened life spans because of delayed treatment-related toxicity, including secondary malignancies and cardiovascular diseases.37While standard-of-care regimens have focused on reducing chemotherapy cycles and radiation dosage, more novel agents are needed to further decrease long-term toxicity in patients. The development and subsequent FDA approval of targeted agents, such as brentuximab vedotin, have significantly added therapeutic options for patients with relapsed/ refractory disease.
Brentuximab vedotin is an ADC that is composed of the monoclonal anti-CD30 antibody linked to monomethyl auristatin E (MMAE). The CD30 antigen, a transmembrane glycoprotein, became an attractive target for therapeutic agents because of its restricted expression in lymphomas, especially in HL and ALCL.38The cytotoxic agent MMAE is a tubulin modifier that blocks cell division. Binding of brentuximab vedotin to CD30 on the cell surface, internalization through a receptor-mediated mechanism, and subsequent release of MMAE in the cytosol are highly effective and induce growth arrest and apoptosis selectively in lymphoma cells.39The initial phase I, dose-escalation study of brentuximab vedotin showed tumor regression in 86% of patients with relapsed/refractory HL and ALCL.40
The pivotal phase II study, which led to FDA approval of brentuximab vedotin in 2011, was completed in patients with relapsed/ refractory HL who had received ASCT. The overall response rate (ORR), the primary endpoint of the study, was 75%, with 34% of patients demonstrating complete remission. Tumor regression was observed in 94% of patients. The median time to treatment response was rapid, just 5.7 weeks, with complete remission achieved in 12 weeks. The median PFS was nearly 6 months for all patients and the median duration of response was more than 20 months for patients with complete response. The median OS was 40.5 months. The safety profile of brentuximab vedotin was acceptable and the most common treatment-related adverse events (AEs) were peripheral sensory neuropathy, nausea, fatigue, diarrhea, and neutropenia.41
The AETHERA trial was a randomized, placebo-controlled, phase III study comparing brentuximab vedotin plus best supportive care (BSC) with placebo plus BSC in patients with HL at high risk of relapse or progression following ASCT. The initial findings of the study were recently published; compared with placebo, brentuximab vedotin improved PFS by 43% and extended PFS by nearly 19 months.42The most common AEs in the brentuximab group were peripheral sensory neuropathy and neutropenia. Based on the results of the AETHERA study, the FDA granted priority review for brentuximab vedotin and in August 2015, granted its approval as a consolidation therapy following ASCT in patients with HL.43
Nivolumab is a fully humanized antiprogrammed cell death 1 (PD-1) antibody that functions as a checkpoint inhibitor. The PD-1 pathway normally acts as a regulator of T-cell activation. When one of the PD-1 ligands, PD-L1 or PD-L2, binds to the PD-1 receptor found on the surface of T cells, the receptor is inactivated. Cancer cells evade an immune response by expressing PD-1 ligands that bind the PD-1 receptor on immune effector cells. Nivolumab binds to PD-L1, preventing binding to the PD-1 receptor, and thus allows T cells to remain active and target the tumor.44In 2014, the FDA granted nivolumab Breakthrough Therapy Designation for the treatment of patients with HL after failure of ASCT or brentuximab vedotin. The designation was largely based on the results of the ongoing phase Ib trial, CheckMate-039. Most patients (87%) treated with nivolumab achieved an objective response and the remaining patients (13%) had stable disease; PFS was 86% after 24 weeks. The safety profile of nivolumab was acceptable, with rash and thrombocytopenia the most common AEs. Grade 3 AEs were noted in 22% of patients.45
Current standard regimens in non-Hodgkin lymphoma
The heterogeneity of NHL subtypes creates complexity in the standard of care. The various subtypes of NHL range from indolent to very aggressive in physiologic behavior and clinical outcome. Thus, the standard treatment regimens described herein will focus on the most prevalent subtypes and those that have received updates with targeted agents in recent years. The most up-to-date standards of care are outlined in the NCCN guidelines.11
Diffuse large B-cell lymphoma
DLBCL accounts for approximately one-third of all NHL cases and is the most commonly diagnosed histologic subtype. The choice of therapy for DLBCL depends on disease stage and IPI score. Clinical presentation of localized disease is fairly uncommon, but combination chemoimmunotherapy plus involved-field radiation is recommended. The standard therapeutic approach for advanced-stage DLBCL is rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy. The addition of rituximab, an anti-CD20 monoclonal antibody, to standard CHOP therapy significantly improved both event-free survival and OS in younger and older patients.46,47Intensification strategies, such as more frequent R-CHOP treatment cycles and ASCT as a first-line treatment, have not shown significant clinical benefit.48,49Thus, R-CHOP remains the gold standard for advanced disease and is being investigated with additional novel agents, such as lenalidomide and ibrutinib, in clinical trials.50,51
Progressive disease after R-CHOP therapy should be tested repeatedly by biopsy to confirm disease recurrence. Most patients should receive combination salvage chemotherapy and subsequent ASCT with successful treatment response. Commonly used salvage chemotherapy regimens, such as rituximab plus ICE and rituximab plus dexamethasone, cisplatin, and cytarabine (DHAP), contain cisplatin and/or gemcitabine agents and produce similar ORRs, event-free survival, and OS.52Additional maintenance therapy after ASCT has not shown significant clinical benefit over no additional therapy.53 Disease progression after ASCT is associated with a very poor prognosis. Relapsed/refractory disease can be treated with allogeneic stem-cell transplantation (allo-SCT) but is not highly recommended for patients with DLBCL because of the likelihood of failure and high nonrelapse mortality rate, especially in patients older than 45 years of age.54
Follicular lymphoma
Follicular lymphoma accounts for about 20% of all lymphomas and is the second most commonly diagnosed lymphoma in the United States. Patients rarely present with stage I or stage II disease. For those who do present with early-stage disease, radiation therapy is recommended and is associated with very good outcomes.55Most patients present with advanced-stage disease, with 70% experiencing bone marrow involvement. Though rare, transformation of follicular lymphoma to a more aggressive lymphoma occurs at a rate of approximately 2% per year.56Clinical management of advanced disease should be assessed based on symptoms and disease bulk. Patients who are asymptomatic and have low tumor bulk might not require therapy until other symptoms appear, such as compromised end-organ function, pancytopenia, or constitutional symptoms, and could be managed through watchful waiting.57
The OS of patients with follicular lymphoma has improved significantly with the incorporation of rituximab into treatment strategies. Compared with watchful waiting, rituximab monotherapy has significantly improved PFS and quality of life.58Generally, patients with low tumor burden and moderate symptoms are initially treated with rituximab followed by a continued rituximab maintenance strategy.59Rituximab plus chemotherapy is a common approach for patients with greater tumor burden. Both bendamustine plus rituximab and R-CHOP or R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone) have produced ORRs greater than 90%.60Chemoimmunotherapy-responsive patients will typically receive ongoing rituximab maintenance therapy, which has been shown to improve the complete response rate and PFS compared with observation alone.61Similar results in complete response rate and PFS have been reported with consolidation radioimmunotherapy.62
Many therapeutic options are available for patients with relapsed/refractory follicular lymphoma, including alternative rituximab-containing chemotherapies, radioimmunotherapy, or treatment with novel agents still in clinical trials. ASCT may also be considered for relapsed patients and has been shown to improve PFS and OS in patients compared with chemotherapy.63Although allo-SCT has shown clinical efficacy in patients with relapsed/refractory disease, a retrospective analysis has demonstrated a significantly greater 3-year OS with autologous SCT than with allo-SCT.64 Recently, idelalisib was approved for use in patients with relapsed/ refractory disease.
Mantle cell lymphoma
Mantle cell lymphoma accounts for 5% to 7% of all lymphomas and has a significant male predominance. As with DLBCL and follicular lymphoma, most patients with MCL present with advanced disease, often with bone marrow, peripheral blood, and gastrointestinal involvement.65While a small subset of patients have indolent disease that can initially be addressed with watchful waiting, most will require treatment at the time of diagnosis.66
Chemoimmunotherapy has been the cornerstone of MCL treatment. Although R-CHOP was the standard of care for many years, recent randomized studies have demonstrated that bendamustine plus rituximab therapy produced improved PFS with less toxicity than R-CHOP as a first-line therapy.67Younger patients may benefit from a more aggressive initial treatment approach, such as with cytarabine plus ASCT or with rituximab plus fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine (R-Hyper-CVAD).68Alternatively, rotating R-CHOP and R-DHAP treatments followed by ASCT during first remission may be implemented.69Most patients with MCL will progress despite the use of ASCT.70The targeted agents bortezomib and ibrutinib have been approved for use in MCL in the frontline and relapsed settings respectively, and ongoing trials are evaluating other therapeutic agents.
Anaplastic large-cell lymphoma
ALCL accounts for 3% to 8% of all lymphomas and 10% to 20% of all childhood lymphomas. While systemic ALK-positive ALCL occurs more frequently in children and young adults and is associated with a better prognosis, ALK-negative disease commonly presents in older adults and is associated with worse outcomes.71Similar to many other types of NHL, patients with ALCL typically present with advanced-stage disease. For the small subset of patients with early-stage disease, a short-course CHOP regimen plus radiotherapy is recommended for both ALK subtypes. A therapy approach similar to that of early-stage DLBCL is acceptable.72
For aggressive ALK-positive ALCL, CHOP, or CHOP-like treatment regimens are appropriate and generally have favorable outcomes in the first-line setting. ALK-negative patients have a variable, and typically worse, response to CHOP therapies.73Alternative treatment options for those patients may include an etoposide-containing regimen, such as CHOEP (cyclophosphamide, doxorubicin, etoposide, vincristine, and prednisone), which has shown superior event-free survival and PFS compared with CHOP in ALK-negative ALCL.74,75Long-term survival rates in patients with ALCL using standard firstline approaches are about 90% and 60% for ALK-positive and ALKnegative patients, respectively.76
Relapsed or refractory ALCL has traditionally been treated with high-dose chemotherapy or ASCT. Salvage rates using this therapeutic approach have been good, with reported 3-year PFS and OS rates of 42% and 53%, respectively, for patients in complete remission. ASCT has also demonstrated clinical superiority to allo-SCT in this subset of patients.77Before the development and approval of the targeted agent, brentuximab vedotin, outcomes were very poor for patients with relapsed/refractory ALCL who were ineligible for transplantation or who failed second-line therapy.73Brentuximab is the most widely studied ADC in ALCL, and it remains the only approved, targeted therapy for systemic relapsed/refractory ALCL.
Chronic lymphocytic leukemia/small lymphocytic lymphoma
Although historically viewed as two distinct diseases, CLL and SLL are now considered the same disease with different clinical manifestations. While most CLL tumors originate in the blood and bone marrow, most SLL tumors originate in the lymph nodes and spleen. CLLs/SLLs make up 5% to 10% of all lymphomas and mainly affect the elderly.78 Parameters that should be assessed before treatment include the clinical stage of disease, symptoms, patient fitness, and specific genetic markers.79Several chromosomal deletions are prevalent in CLL/SLL. Two such deletions, del(11q) and del(17p), are found in approximately 25% and in 5% to 8% of chemotherapynaïve patients, respectively.78These deletions are notable because NCCN guidelines specifically stratify treatment regimens based on their presence or absence in CLL/SLL.11
Alkylating agents, such as chlorambucil, are the gold standard for initial frontline therapy and have been for several decades.80Even with the advent of monoclonal antibody therapies and other targeted agents, chlorambucil remains a viable treatment option, especially in the elderly or unfit patients. Patients with symptomatic, advanced disease and good physical fitness (normal creatinine clearance and a low cumulative illness rating scale score) can be treated using combination therapies such as fludarabine, cyclophosphamide, and rituximab (FCR). Patients with advanced disease who are unfit or somewhat physically impaired or who have the del(11q) chromosomal deletion can be treated with chlorambucil plus an anti-CD20 monoclonal antibody such as rituximab, obinutuzumab, or ofatumumab, or alternatively, with bendamustine plus rituximab. In patients with symptomatic disease and the del(17p) chromosomal deletion, treatment with either the kinase inhibitors, idelalisib or ibrutinib, alone or in combination with rituximab, is recommended.11,78These inhibitors, especially ibrutinib, have demonstrated significant, long-term clinical efficacy in high-risk patients.81,82
For relapsed/refractory disease, the first-line treatment can be repeated if the progression or relapse occurs 2 years or more after the remission. For relapse that occurs within 2 years after response to first-line therapy, several treatment options are available. Treatment with idelalisib or ibrutinib should be offered to all patients, including those with or without the del(11q) and del(17p) mutations. Alternatively, a chemoimmunotherapy, such as FCR, bendamustine plus rituximab, or alemtuzumab plus rituximab, can be offered.11Allogeneic SCT may be offered to high-risk patients with CLL/SLL who are treatment refractory to purine analogs, have a short response to first-line therapy, or harbor the del(17p) mutation.83Lastly, as with most relapsed/refractory disease, experimental agents being studied in clinical trials should be suggested.
Targeted regimens recently approved in non-Hodgkin lymphoma
In recent years, the development and approval of several targeted agents have greatly improved clinical outcomes for patients with NHL. Advances have been achieved in both a frontline setting, as with obinutuzumab and bortezomib, and in relapsed/refractory disease, as with brentuximab vedotin, ibrutinib, idelalisib, and lenalidomide.
In 2011, brentuximab vedotin was granted FDA approval for patients with systemic ALCL who had failed at least one prior multiagent chemotherapy. This approval was made concurrently with the approval for HL. Because expression of CD30 is high in ALCL, investigators hypothesized that targeted therapy with an anti-CD30 monoclonal antibody would be efficacious. However, early studies using a naked monoclonal antibody showed poor antitumor activity (ORR, 17%).84The development of the ADC with the addition of MMAE to the anti-CD30 antibody significantly improved efficacy in preclinical and early-phase clinical trials.40Approval of brentuximab vedotin was based largely on a subsequent, multicenter, phase II trial. Of the 58 patients who received therapy, 86% achieved an objective response, with 57% achieving a complete response. Brentuximab vedotin showed efficacy in both ALK-positive and ALK-negative subgroups; patients who were ALK positive had an ORR of 81%, with a complete response rate of 69%. Similarly, patients who were ALK-negative had an ORR of 88%, with a complete response rate of 52%. The median response duration was 12.6 months and the estimated median PFS was 13.3 months with no significant differences in response based on ALK expression. The most common grade 3 or 4 AEs were neutropenia, thrombocytopenia, and peripheral sensory neuropathy that occurred in 21%, 14%, and 12% of patients, respectively.85In a 3-year follow-up study, the median response duration for patients who had a complete response was 26.3 months and nearly 50% remained in remission at the time of analysis.86Ongoing clinical trials are evaluating brentuximab vedotin in a frontline setting as well as in salvage therapy after relapse.
Obinutuzumab is a third-generation anti-CD20 antibody. In 2013, the FDA approved obinutuzumab for use in combination with chlorambucil for treatment-naïve CLL/SLL. Approval of obinutuzumab in the frontline setting was based on a large, randomized, multicenter, phase III trial that compared obinutuzumab plus chlorambucil and rituximab plus chlorambucil versus chlorambucil monotherapy. Both combination therapies significantly prolonged PFS and improved the ORR compared with monotherapy. Additionally, the obinutuzumab-chlorambucil regimen improved the complete response rate by 13% and significantly improved PFS compared with the rituximab-chlorambucil regimen. Grade 3 or 4 infusionrelated reactions were more common with the obinutuzumab arm than with the rituximab arm.87
Ibrutinib is a potent and irreversible inhibitor of the Bruton tyrosine kinase (BTK) that binds near the active site of the protein; BTK is important for B-cell activation and B-cellmediated signaling. Overexpression of BTK is associated with several B-cell NHLs, including DLBCL, MCL, follicular lymphoma, and CLL/SLL. Ibrutinib has been approved for use in patients with MCL or CLL/SLL who have received at least one prior therapy. Approval of ibrutinib for the treatment of MCL was based largely on the results of a multicenter, phase II trial in patients with relapsed/refractory disease. Ibrutinib was well tolerated, with rare incidence of grade 3 AEs, and nearly 70% of patients responded to treatment. Although the median OS was not reached, the estimated rate of OS was 58% at 18 months, with median response duration and PFS at 17.5 months and 13.9 months, respectively. Approval for patients with CLL/ SLL was based on a multicenter, phase Ib-II study in patients with refractory disease. The AEs were mostly grade 1 or 2 and transient. The ORR was 71% for both doses tested, and the estimated PFS and OS rates were 75% and 83%, respectively, after 26 months.82
Idelalisib is a small-molecule inhibitor of PI3K-delta, an isoform of PI3K that is overexpressed in hematopoietic malignancies. By inhibiting activation of the PI3K-signaling pathway and production of the downstream second messenger PIP3, idelalisib blocks tumor cell proliferation, motility, and survival.88Phase I and II studies published concurrently in 2014 have shown acceptable safety and favorable antitumor activity of idelalisib in patients with indolent NHL, relapsed/ refractory CLL, and MCL.89-92A phase III study demonstrated that idelalisib in combination with rituximab significantly improved response rate, PFS, and OS compared with rituximab plus placebo in patients with CLL/SLL unable to undergo chemotherapy.93The incidence of serious AEs was similar between idelalisib plus rituximab and rituximab plus placebo.93Based on these studies, the FDA approved idelalisib in July 2014 for the treatment of relapsed/refractory CLL when used in combination with rituximab and in patients with relapsed/refractory follicular lymphoma or SLL who have received at least two previous systemic therapies.
Bortezomib is a proteasome inhibitor that blocks functional proteolysis by the proteasome and subsequently disrupts intracellular signaling pathways, primarily through inhibition of nuclear factor (NF)-κB. The resulting toxic effects are apoptosis, cell cycle arrest, and inhibition of angiogenesis. In 2006, the FDA approved bortezomib for treatment in patients who had received at least one priory therapy, representing the first drug approval specifically indicated for MCL.94In 2014, the approval was updated to include previously untreated, or treatment-naïve, patients with MCL. The extended approval was based primarily on a phase III, randomized trial in treatment-naïve patients who received either bortezomib plus rituximab, cyclophosphamide, doxorubicin, and prednisone (VcR-CAP) or standard R-CHOP therapy. Treatment with the bortezomib regimen improved PFS by more than 10 months and improved 4-year OS by 10% compared with R-CHOP. The incidence of grade 3 or higher AEs was high but was similar between treatment groups.95
Lenalidomide is an immunomodulatory agent that inhibits TNF-α production, stimulates T cells, and reduces circulating levels of the angiogenic factors vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Recently, lenalidomide has been implicated in the ubiquitin-proteasome pathway.96Clinical trials evaluating the efficacy of lenalidomide have demonstrated superior clinical benefit, namely higher and more durable responses, in patients with MCL than in other NHL subtypes.97,98The pivotal study of lenalidomide, the MCL-001 (EMERGE) phase II, multicenter trial, investigated lenalidomide in patients who had failed prior chemotherapy and bortezomib therapy. Patients exhibited a rapid response time (2.2 months). The median duration of response was 16.6 months and the median OS was 19.0 months. Neutropenia, thrombocytopenia, and anemia were the most common grade 3 or 4 AEs.99These durable responses led to FDA approval of lenalidomide in June 2013 for patients with relapsed/refractory MCL.
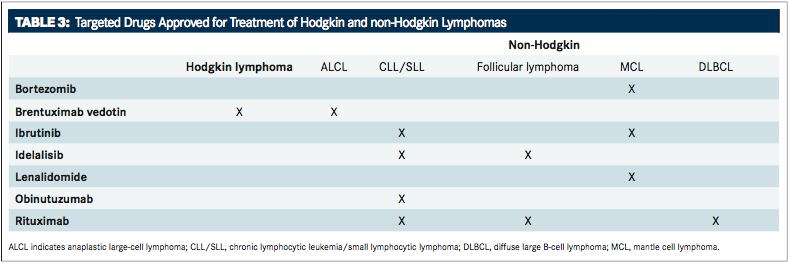
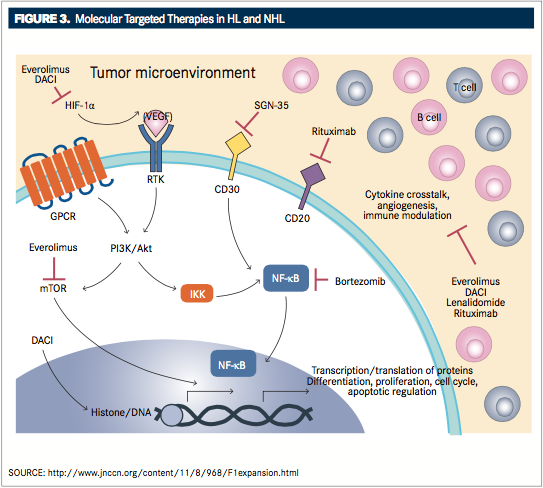
FIGURE 3andTABLE 3provide an overview of molecular-targeted therapies approved for HL and NHL.