Evolving Paradigms in Lymphoma: Overview, Epidemiology, Detection, Diagnosis, and Staging
This feature covers the "I. Introduction: Overview and Epidemiology" and the "II. Detection, Diagnosis, and Staging" sections of the current Evolving Paradigms in Lymphoma issue.
I. Introduction: Overview and Epidemiology
Lymphomas are cancers of the immune system. While 10% of all lymphomas are classified as Hodgkin lymphoma (HL), the remaining 90% are classified as non-Hodgkin lymphoma (NHL). Treatment using targeted therapies has significantly improved outcomes for patients in recent years. This review focuses on the use of targeted agents in the post-transplant setting and changing paradigms in the frontline setting. Lymphoma detection, diagnosis, and staging will be examined, as well as risk stratification and current standard therapeutic options.
Hodgkin Lymphoma
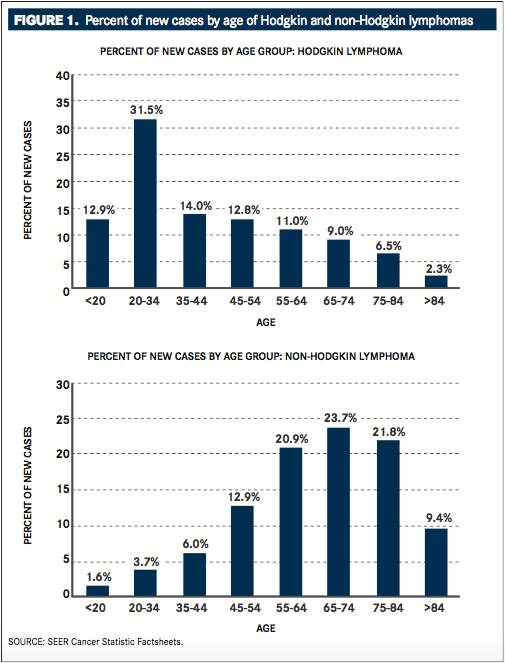
Hodgkin lymphoma is a relatively rare type of cancer. In the United States, HL will account for an estimated 9050 new cancer cases each year, or 0.5% of all new cancer cases, resulting in an estimated 1150 deaths in 2015.1The annual incidence of new cases is 2.7 per 100,000 people per year. Hodgkin lymphoma is most commonly diagnosed in people 20 to 34 years of age, with a median age of diagnosis of 38 years (FIGURE 1, TOP).2 Hodgkin lymphoma is classified as either classical HL, which accounts for 95% of HL cases,
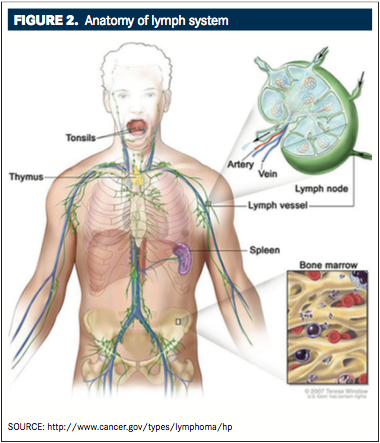
or nodular lymphocyte-predominant, which accounts for the remaining 5% of cases. Classical HL is further subdivided into the four subtypes: nodular sclerosis, mixed cellularity, lymphocyte depleted, and lymphocyte rich.3While the nodular lymphocyte-predominant type of HL is not associated with the Epstein-Barr virus, all classical HL subtypes have an intermediate to strong association with the virus.4At a histologic level, HL is characterized by the presence of mononuclear Hodgkin cells and multi- nucleated Reed-Sternberg cells that have a B-cell origin.3Hodgkin lymphoma can develop in any part of the lymphatic system, most commonly in the lymph nodes and less commonly in the spleen, thymus, tonsils, and bone marrow (FIGURE 2). Risk factors for HL include younger age, male gender, previous infection with the Epstein-Barr virus, and a positive diagnosis of HL in a first-degree relative.4
Non-Hodgkin Lymphoma
Non-Hodgkin lymphoma is the seventh most common cancer in the United States, accounting for approximately 4% of all new cancer cases. In 2015, an estimated 71,850 new cases of NHL will be diagnosed, resulting in an estimated 19,790 deaths.1The annual incidence of new cases of NHL is 19.7 per 100,000 people per year. It is most commonly diagnosed in people 65 to 74 years of age, with a median age of diagnosis of 66 years (FIGURE 1, BOTTOM). Like HL, NHL is more commonly diagnosed in men than in women.5Among the many subtypes of NHL, the prevalence varies significantly by geographic region.6
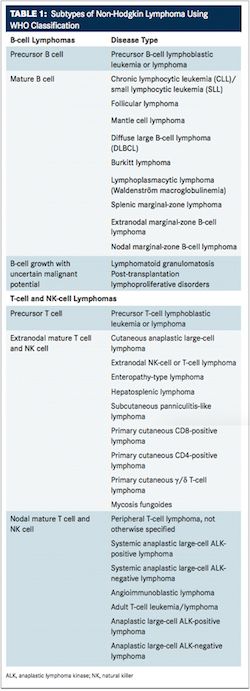
As with HL, NHL can develop in almost any part of the lymphatic system but typically occurs in the lymph nodes. Among this heterogeneous group of cancers, 85% to 90% of subtypes derive from B lymphocytes and the remaining subtypes develop from T lymphocytes or from natural killer lymphocytes.7These subtypes also range from indolent lymphomas, such as follicular lymphoma, to more aggressive lymphomas, such as Burkitt lymphoma and diffuse large B-cell lymphoma (DLBCL).TABLE 1lists the subtypes of NHL based on the World Health Organization (WHO) classification of tumors in hematopoietic and lymphoid neoplasms.8
II. Detection, Diagnosis, and Staging
For both HL and NHL, patients usually present with painless lymphadenopathy, often in the cervical or supraclavicular regions. In more than 50% of cases of HL, a mediastinal mass is present that is either asymptomatic or presents as cough, dyspnea, or superior vena cava obstruction. For both diseases, the clinical presentation depends on the site involvement, pathophysiology of the lymphoma subtype, and presence or absence of B symptoms that include night sweats, loss of bodyweight greater than 10%, and fever.4,9
Guidelines for the diagnosis and staging for HL and NHL have been developed by the National Comprehensive Cancer Network (NCCN) and are well accepted.10,11The standard for detection of HL is excisional biopsy followed by evaluation by immunohistochemistry (IHC).10For diagnosis of NHL, a core needle biopsy or fine needle aspiration is obtained. However, this sample is often not sufficient for an initial diagnosis because of the need for additional molecular testing that may be required to obtain a differential diagnosis. Such testing may include flow cytometry, polymerase chain reaction (PCR) analysis, fluorescence in situ hybridization (FISH), or IHC. Most NHL subtypes require immunophenotyping using an IHC panel or flow cytometry analysis to detect cell surface markers, such as CD5, CD10, CD19, CD20, and CD23, or other distinguishing markers, such as cyclin D1, B-cell lymphoma-2 (BCL-2), and Ki-67. Most NHL subtypes have a unique immunophenotype profile. Essential workup procedures for either disease may include assessment of B symptoms, a complete blood count, comprehensive metabolic panel, bone marrow biopsy, and various imaging methods.11
Computed tomography (CT) and positron emission tomography (PET) are commonly used for disease staging and assessment. While imaging of the neck, chest, abdomen, and pelvis for staging has been traditionally performed using contrast-enhanced CT, the use of PET scanning with 18F-fluorodeoxyglucose (18F-FDG) has risen in recent years because of its increased accuracy, its ability to delineate margins for radiotherapy, and its ability to provide baseline, interim, and end-of-treatment assessments.12,13In HL, 18F-FDG PET is particularly sensitive for detecting focal bone-marrow infiltration and has the potential to reduce the frequency of invasive trephine biopsies needed for staging.14Subtypes of NHL have varied affinity for detection using 18F-FDG PET. For example, follicular, DLBCL, and mantle cell lymphoma (MCL) are consistently 18F-FDG PET avid, while small lymphocytic lymphoma (SLL), T-cell, and extranodal marginal-zone lymphomas demonstrate variability with 18F-FDG PET avidity. In NHL subtypes that are consistently avid, 18F-FDG PET scanning detects with 80% sensitivity and 90% specificity.12
When used for diagnostic purposes, 18F-FDG PET can result in disease upstaging and has been shown to be superior to CT in sensitivity of detection.15,16Upstaging from early-stage to advanced-stage disease, which occurs in 10% to 15% of patients with HL, has the potential to modify clinical management of the disease.16,17Perhaps the greatest benefit of 18F-FDG PET is its use in the interim analysis after initial cycles of chemotherapy to determine which patients need escalation or de-escalation of treatment. In HL, assessment after two cycles of chemotherapy has shown to be a better predictor of outcomes than traditional risk stratification analysis.17,1818F-FDG PET uptake is quantified using the 5-point Deauville scale, which compares the lesion uptake value with that of the liver or mediastinum.19Staging of both HL and NHL is made using the Ann Arbor system and Cotswold modification (TABLE 2).20,21