Evolving Paradigms in Lymphoma: Emerging Therapies in Clinical Trials and Conclusions
This feature covers the "V. Emerging Therapies in Clinical Trials" and the "Conclusions" sections of the current Evolving Paradigms in Lymphoma issue.
V. Emerging Therapies in Clinical Trials
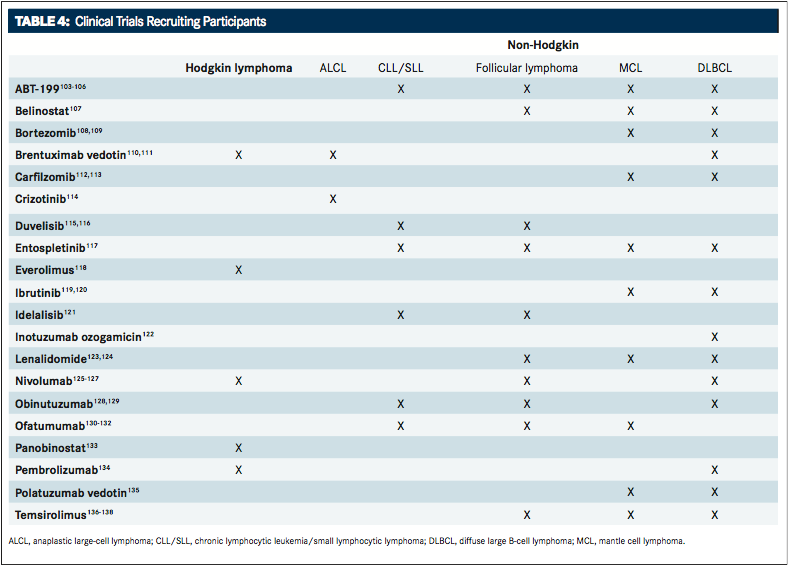
Several agents are in clinical trials for the treatment of HL and NHL. A greater understanding of the molecular mechanisms driving these diseases has prompted the development of novel, highly active agents. Clinical research efforts aim to improve cure rates while reducing treatment-related toxicity. Some of the most promising agents, which are being studied in the frontline and in relapsed/refractory settings, include monoclonal antibodies, antibody-drug conjugates, and small molecule inhibitors.TABLE 4provides an overview of clinical trials using targeted agents that are now recruiting participants.
Monoclonal antibodies
Blinatumomab is a bispecific anti-CD19/CD3 antibody that is ap- proved for the treatment of relapsed/refractor acute lymphoblas- tic leukemia. It belongs to a class of molecules known as BiTE, or bispecific T-cell engager. These molecules recruit and activate endogenous T cells to target CD19-postive B cells and therefore act as a bridge between a cancer cell and a T cell. Bispecific antibod- ies, such as blinatumomab, are clinically advantageous for their low effector-to-target ratio and high tissue penetration.100Recent clinical trials have focused on the efficacy of blinatumomab in B- cell NHL and B-cell acute lymphoblastic leukemia.
In an initial phase I trial in patients with NHL, 10% achieved com- plete response and 18% achieved a partial response with blinatu- momab therapy over 4 to 8 weeks with tumor cell clearance from the liver and bone marrow. Patients demonstrated high rates of grade 3 or higher AEs, including lymphopenia, elevated C-reactive protein levels, leukopenia, neutropenia, and thrombocytopenia. In a more recent phase I study, investigators found that circulating B cells were completely depleted using a modest concentration of blinatumomab.101A phase II multicenter trial of patients with re- lapsed/refractory DLBCL is ongoing. Investigators will assess ORR (complete or partial) during the first treatment cycle using the Che- son criteria from data obtained using CT and PET scans.102
Rituximab is an anti-CD20 antibody that is approved for use with standard chemotherapy regimens as a frontline therapy in patients with follicular lymphoma, CLL/SLL, and DLBCL. Ongoing studies with rituximab are focused on its use in the frontline setting in com- bination with other multiagent chemotherapies or immunomodula- tory agents. Initial phase II clinical trials in patients with HL were promising and demonstrated event-free survival and OS rates of 83% and 96%, respectively, when combined with ABVD.139Another phase II trial completed at the MD Anderson Cancer Center has in- vestigated rituximab in combination with lenalidomide in patients with follicular lymphoma, marginal zone lymphoma, and SLL. An ORR of 90% with a complete response of 63% was observed. Among the follicular, marginal zone, and SLL NHL subtypes, complete re- sponse rates of 87%, 67%, and 23% were achieved, respectively. Le- nalidomide plus rituximab was well tolerated, with neutropenia as the most common grade 3 or 4 AE.140To date, researchers at the German Hodgkin Study Group completed the only phase III trial of rituximab in combination with escalated BEACOPP in patients with HL; the addition of rituximab did not improve OS or PFS after 3 years, and more than 90% of patients experienced grade 4 toxic- ity.141Investigators at the Sidney Kimmel Comprehensive Cancer Center are recruiting participants in a pilot study of rituximab and brentuximab vedotin in patients with chemosensitive relapsed HL. One-year failure-free survival is the primary outcome of the study.142
Ofatumumab is another humanized anti-CD20 antibody that is approved for the treatment of relapsed/refractory CLL/SLL.143In preclinical models of MCL, ofatumumab demonstrated superior- ity to rituximab, even in a rituximab-resistant setting.144Clini- cal trial data of ofatumumab for treatment of MCL are limited.145Therefore, two phase II trials are being conducted to determine the effect of ofatumumab in MCL. The first study, conducted at the Memorial Sloan Kettering Cancer Center, will evaluate the effect of ofatumumab alone or in combination with the alkylating agent bendamustine in MCL. The primary outcomes are single-agent and multiagent efficacy, and secondary outcomes include OS, PFS, remission duration, and response duration.131Roswell Park Can- cer Institute is conducting the second phase II trial, which will evaluate the effectiveness of ofatumumab in combination with hy- perfractionated cyclophosphamide, doxorubicin, vincristine, and dexamethasone (O-HyperCVAD) and alternating with ofatumumab in combination with cytarabine and methotrexate. Investigators will determine the percentage of patients who achieve a complete response as the main outcome.146In the large phase III ORCHARRD study of patients with DLBCL or refractory follicular lymphoma, ofatumumab plus dexamethasone, cytarabine, and cisplatin failed to demonstrate clinical superiority in PFS when compared with rituximab plus dexamethasone, cytarabine, and cisplatin.147
Epratuzumab is a humanized IgG1 antibody that targets the B- cell antigen CD22, which regulates cellular adhesion and B-cell activation. CD22 is an attractive drug target because most B-cell NHLs express it at high levels. The clinical utility of epratuzumab has been evaluated in combination with standard chemotherapy regimens and other monoclonal antibodies. In one phase II study, investigators compared the efficacy of epratuzumab plus R-CHOP (ER-CHOP) with standard R-CHOP therapy in patients with DLB- CL. Those who received ER-CHOP showed improved PFS compared with R-CHOP alone.148A phase II trial that evaluated the combi- nation of epratuzumab with rituximab immunotherapies demon- strated complete and partial responses above 40%, with 60% of patients remaining in remission at the 3-year follow-up, levels that are comparable to chemotherapy-containing regimens.149An ongo- ing phase II study will evaluate the efficacy of radiolabeled epratu- zumab in combination with veltuzumab, an anti-CD20 antibody, in patients with aggressive, refractory NHLs, including DLBCL, MCL, and follicular lymphoma. Safety and dose-limiting toxicity are the primary outcomes of the study.150
Antibody-drug conjugates
Clinical trials are investigating brentuximab vedotin in a frontline setting. Two trials, ECHELON-1 and ECHELON-2, are investigating brentuximab vedotin in combination with standard chemothera- peutic combination therapies. The ECHELON-1 trial is an open- label, multicenter, phase III study comparing brentuximab vedotin plus AVD with ABVC in patients with advanced HL. Modified PFS is the main outcome of the study.151The ECHELON-2 trial is a dou- ble-blind, multicenter, randomized phase III study comparing the safety and efficacy of brentuximab vedotin plus CHP (cyclophos- phamide, hydroxydaunomycin/doxorubicin, and prednisone) with standard-of-care CHOP in patients with CD30-positive mature T- cell lymphomas, including ALCL; PFS is the main outcome of the study.152
Other phase II studies have investigated the efficacy of bren- tuximab vedotin as first-line salvage therapy prior to ASCT in patients with relapsed/refractory HL. Standard salvage therapies such as ICE or GCD are effective but have significant toxicities. In two independent studies, investigators determined whether brentuximab vedotin, used as salvage therapy, would negate the need for additional ICE therapy prior to ASCT. Interim analysis was performed by PET scan. The results indicated that 27% to 52% of patients were PET-negative after brentuximab vedotin treatment and proceeded directly to ASCT, eliminating ICE che- motherapy.153,154Another phase II trial assessed brentuximab vedotin in combination with bendamustine in patients with re- lapsed/refractory HL. The complete remission and ORR (both complete and partial) rates were 82% and 94%, respectively. The safety profile was manageable.155Ongoing phase III studies are exploring brentuximab versus physician’s choice of treatment (methotrexate or bexarotene) in patients with CD30-positive cutaneous T-cell lymphoma156 and brentuximab plus combina- tion chemotherapy versus combination chemotherapy alone in patients with HL who are 2 to 18 years of age.157
Inotuzumab ozogamicin is a humanized anti-CD22 IgG4 mono- clonal ADC covalently linked to the antitumor agent calicheami- cin. Inotuzumab ozogamicin works through an internalization mechanism whereby it binds to CD22 on the cell surface, is rapid- ly internalized, and delivers the antitumor drug internally, result- ing in double-stranded DNA breaks and apoptosis.158Completed phase I and phase II trials have shown moderate response rates with inotuzumab ozogamicin alone and with rituximab in patients with follicular lymphoma and DLBCL.159-161The Oncology Institute of Southern Switzerland is conducting an open-label phase I trial to study the effects of inotuzumab ozogamicin in combination with the mammalian target of rapamycin (mTOR) inhibitor, temsiroli- mus, in patients with CD22-positive B-cell NHLs. The primary out- come of the study is determining the safety profile, including AEs and the maximum tolerated dose.162University College, London, is conducting a randomized phase II trial comparing inotuzumab ozogamicin plus rituximab and CVP with gemcitabine plus ritux- imab and CVP as first-line treatments in patients with DLBCL who are not suitable for anthracycline-containing chemotherapy. The primary outcome of the study is PFS.122 Other ADCs in clinical phase I or II trials in patients with NHL include polatuzumab ve- dotin, pinatuzumab vedotin, SAR3419, and SGN-CD19A.158
Checkpoint inhibitors
Nivolumab has not been approved for patients with HL; however, on- going phase II trials are under way. In the CheckMate-205 study, in- vestigators will determine the best ORR of either partial remission or complete remission and the duration of response with nivolumab in patients with HL.125Two additional phase II studies, CheckMate-139 and CheckMate-140, which are recruiting patients, will evaluate nivolumab in patients with NHL. CheckMate-139 will evaluate the effectiveness of nivolumab in patients with relapsed or refractory DLBCL after failure of ASCT or failure of at least two previous che- motherapy regimens, and CheckMate-140 will evaluate the effective- ness of nivolumab in patients with relapsed or refractory follicular lymphoma.126,127The ORR is the primary outcome for both studies.
Pembrolizumab is another antiPD-1 monoclonal antibody. It is approved in the US for the treatment of metastatic melanoma. A phase Ib trial, KEYNOTE-013, is recruiting participants to de- termine the safety, tolerability, and efficacy of pembrolizumab in patients with several different types of hematologic malignancies, including those with HL, mediastinal large B-cell lymphoma, PD- L1–positive NHL, follicular lymphoma, and DLBCL. The main out- comes include the number of participants that experience AEs, the number of participants that discontinue treatment because of AEs, ORR, and complete remission rate.163The Dana-Farber Cancer In- stitute is conducting a phase II trial to study the clinical efficacy of pembrolizumab in patients with relapsed/refractory HL or DLBCL when provided after ASCT. Investigators will determine the PFS 18 months after ASCT as the primary outcome.134
Small molecule inhibitors
Everolimus is an mTOR inhibitor that is approved for the treat- ment of breast, pancreatic, and kidney cancers and for the treat- ment of subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Everolimus functions by binding to mTOR and preventing downstream activation of the PI3K/Akt signaling pathway, resulting in decreased tumor cell growth, an- giogenesis, and transcription.164Several clinical trials are inves- tigating everolimus in combination with other therapeutic agents in both HL and NHL. Most trials are in phase I or phase II. The first phase II trial is investigating everolimus and panobinostat, an HDAC inhibitor, in patients with relapsed/refractory DLBCL. The primary outcomes are ORR and the response rate predicted by molecular signatures developed from preclinical models.165The second phase Ib/II trial is investigating everolimus and AEB071, a protein kinase C inhibitor, in DLBCL with either the CD79 mutant or the ABC subtype. The primary outcomes are determining the dose-limiting toxicities and maximum tolerated dose.166The third phase II study, being conducted by investiga- tors at the Sidney Kimmel Comprehensive Cancer Center, will examine the combination of everolimus with rituximab as main- tenance therapy after high-dose chemotherapy in patients with B-cell lymphomas and HL. The primary outcomes are safety and tolerability.
Temsirolimus is an mTOR inhibitor that is approved for use in kidney cancer. Treatment with temsirolimus was shown to signifi- cantly improve PFS and objective response rate relative to the in- vestigator’s choice of therapy in a phase III trial of patients with relapsed/refractory MCL.167Therefore, in a phase IV trial, investi- gators are comparing the safety and efficacy of two different doses of temsirolimus; PFS is the primary outcome of the study.168
An ongoing phase III clinical trial is recruiting patients to in- vestigate the safety and efficacy of idelalisib in combination of rituximab in patients with indolent NHL who were previously treated. The primary outcome is PFS and secondary outcomes include ORR, complete response rate, OS, and lymph node re- sponse rate.169
Duvelisib is an inhibitor of the delta and gamma isoforms of PI3K and, like idelalisib, has an affinity for hematologic cancer cells. Early clinical trials investigating duvelisib in patients with relapsed/refractory indolent NHL and T-cell lymphoma have shown ORRs above 50% and acceptable safety profiles, although transaminitis is a common AE.170,171In one phase III trial, investi- gators are comparing the efficacy of duvelisib monotherapy with ofatumumab monotherapy in patients with relapsed/refractory CLL/SLL; PFS is the primary outcome.116Another phase III trial is assessing the safety and efficacy of duvelisib in combination with rituximab versus rituximab monotherapy in patients with follicular lymphoma who are not suitable for chemotherapy. The primary outcome is PFS.115
ABT-199 is a second-generation inhibitor of BCL-2, an important regulator of apoptosis. BCL-2 is an antiapoptotic protein that is overexpressed in several cancers. ABT-199 mimics the BH3 pro- tein, a ligand of BCL-2 that activates apoptosis, and thus restores death-promoting processes in tumor cells. In an ongoing phase I dose-escalation trial of patients with various types of relapsed/ refractory NHL, the ORR was near 50% and the most notable re- sponses were in patients with MCL and Waldenström macroglobu- linemia. Responses were observed across a wide range of doses.172Clinical trials are evaluating the safety and efficacy of ABT-199 in combination with other therapeutic agents. An open-label, phase III, randomized study will evaluate the efficacy of ABT-199 plus rituximab and bendamustine plus rituximab in patients with relapsed/refractory CLL/SLL. The primary outcome is PFS.103A second multicenter, randomized, phase III trial will compare the safety and efficacy of ABT-199 plus obinutuzumab (an anti-CD20 antibody) with ABT-199 plus obinutuzumab and chlorambucil in patients with CLL/SLL. PFS is the primary outcome of the study.173
Conclusions
The treatment of HL and NHL is rapidly evolving. Continual under- standing of the genetic and molecular profiles of lymphomas will promote better risk stratification and, simultaneously, enhance the development of novel, targeted agents. Several new agents have been approved in recent years and ongoing clinical trials have demonstrated promising results in both the frontline setting and in relapsed/refractory disease. The integration of these new agents into standard-of-care practices is key to improving the prognosis, clinical outcomes, and quality of life for patients.
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More





