Daratumumab Shifts Approach in Newly Diagnosed Multiple Myeloma
In a comparison of 2 live events from the Targeted Oncology Case-Based Roundtable series, we look at how adding daratumumab to therapy for patients with newly diagnosed disease who cannot receive a transplant has shifted the approach to treatment.
Treatments for patients with newly diagnosed multiple myeloma become limited when these patients are ineligible for autologous stem cell transplant (ASCT), but as evidence for new therapies and the use of quadruplet therapy has grown, so too have the approved options for this population. Chief among these is the addition of daratumumab (Darzalex) to lenalidomide (Revlimid) and dexamethasone (DRd), which has shown long-term survival results in these patients and important subgroups.1-3
Although daratumumab continues to prove its role in this treatment landscape, the use of quadruplet regimens remains a topic of debate. This debate has extended into the National Comprehensive Cancer Network (NCCN) guidelines, which give bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone (VRd) and DRd a category 1 preferred regimen designation, but do not currently list quadruplets under preferred regimens.4
Quadruplet regimens are part of the NCCN’s other recommendations regimens, and the category 1 quadruplet recommended for transplant-ineligible patients is daratumumab, bortezomib, melphalan, and prednisone (D-VMP), whereas daratumumab, cyclophosphamide, bortezomib, and dexamethasone is not category 1. However, physicians continue to assess the individual needs of the patient and utilizing the best treatment for them, especially in a population who can be of advanced age and have frailty issues.
In 2 separate Case-Based Roundtable events, physicians took up the discussion on treatments for those who are ineligible for transplant by focusing on the case of an 81-year-old patient with International Staging System III multiple myeloma λ with a 50% to 60% standard risk based on fluorescence in situ hybridization (FISH). The patient had an ECOG performance status of 2 and an International Myeloma Working Group (IMWG) frailty index categorization of intermediate-frail, and therefore was considered ineligible for transplant.
Led by Louis Williams, MD, and Alfred Garfall, MD, physicians from the Cleveland, Ohio, and New Jersey-Pennsylvania areas, respectively, discussed how they would make their treatment decisions in regard to triplet therapies with daratumumab. They also discussed what these new data mean for their more vulnerable patients.
When to Use Daratumumab Triplet and Quadruplet Therapy
Triplet therapies have become the standard of care and preferred treatment for many patients with multiple myeloma, but now physicians have a choice regarding which regimens to use.
In a literature review of available data on triplet therapies, researchers wrote that among 122 publications, the data showed the daratumumab-containing regimens DRd and D-VMP, along with VRd, had a higher likelihood than the doublet therapy of lenalidomide and dexamethasone of resulting in a better rate of progression-free survival (PFS)—the primary end point—and overall survival (OS).5 Compared with Rd, HRs favored the triplet and quadruplet therapy over the doublet therapy for PFS at 0.53 with DRd, 0.57 with D-VMP, and 0.77 with VRd. Similar results were seen for OS with HRs of 0.68, 0.77, and 0.78, respectively. Moreover, the researchers concluded that DRd had the highest likelihood of being the most effective therapy in terms of a patient’s survival.
This bore out in the results of the phase 3, randomized, MAIA trial (NCT02252172).6 In an interim analysis of 737 patients with newly diagnosed multiple myeloma who were ineligible for ASCT, the initial findings showed that those given DRd (n = 368) had a higher probability of being alive without disease progression at 30 months than those who received Rd (n = 369), at 70.6% (95% CI, 65%-75.4%) in the DRd group vs 55.6% (95% CI, 49.5%-61.3%) in the Rd group (HR, 0.56; 95% CI, 0.43-0.73, P <.001). Moreover, 47.6% of patients had a complete response (CR) or better in the DRd group vs 24.9% in the Rd group (P <.001).
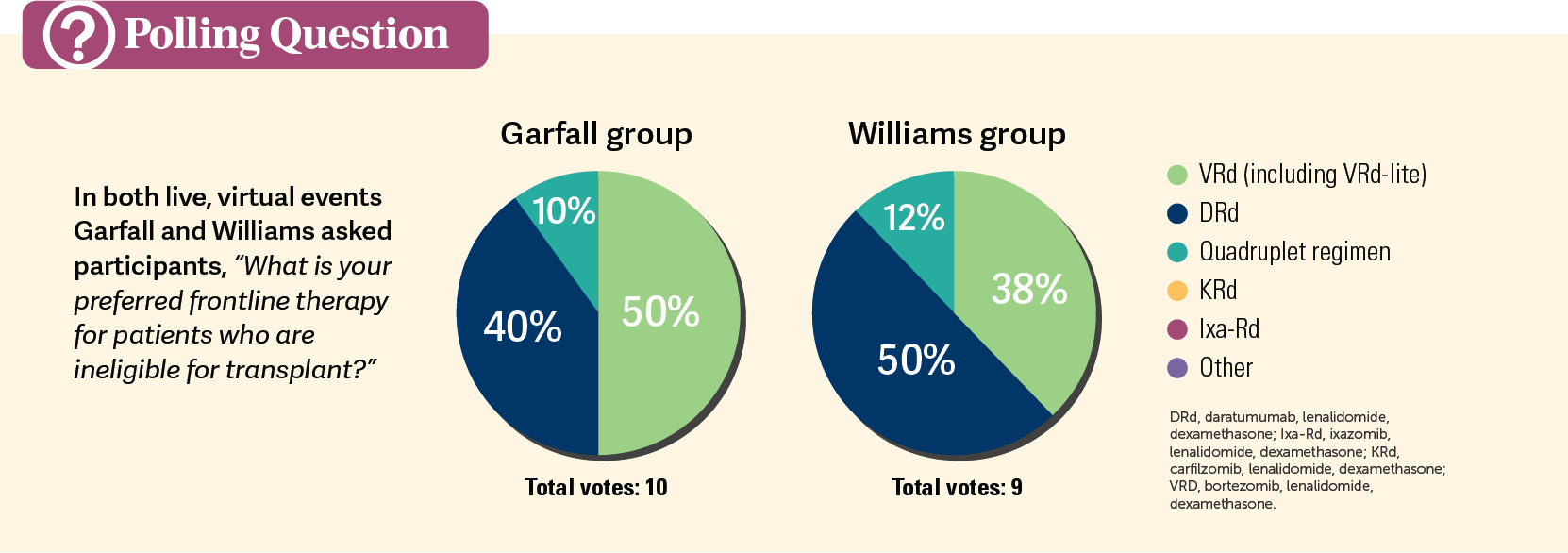
When asked which therapy was their preferred frontline therapy in this patient population, physicians in both groups were split between regimen (Polls). In Garfall’s group, 50% preferred VRd vs 40% who chose DRd, whereas in Williams’ group 50% preferred DRd vs 38% who chose VRd between the 2 triplet regimens. Also, in each group, 1 physician preferred to start right away with a quadruplet therapy.
“I love DRd in transplant-ineligible patients; I think it works extremely well and rapidly, but I do have to have discussions [with patients] on the convenience factor of it,” explained Kevin Rakszawski, MD, a physician from the Garfall group and medical director of the Schwartz Center for Compassionate Healthcare. “For some of my patients, it’s more of an issue [because] some of my patients travel quite a distance, so they prefer Rd to traveling frequently [for daratumumab]. But I do try to use daratumumab whenever I can.”
Long-Term Use of Daratumumab
A few years later, the MAIA researchers looked at the updated results.1,2 At a median follow-up of 64.5 months, PFS, overall response rate (ORR), and minimal residual disease (MRD) negativity still favored daratumumab, as well as OS at 73.6 months median follow-up.
The median PFS at the time of the updated results was 61.9 months with DRd vs 34.4 months with Rd (HR, 0.55; 95% CI, 0.45-0.67; P <.0001). The 60-month PFS rates were 52.1% vs 29.6% for DRd vs Rd, respectively. The median OS was not reached for patients given DRd compared with 64.1 months in patients receiving Rd (HR, 0.65; 95% CI, 0.52-0.80; P <.0001). In the DRd group, the 60-month OS rate was 66.7% vs 53.7% in the Rd group.
In terms of MRD-negativity rates, 32.1% of patients in the DRd arm of the MAIA trial met this secondary end point compared with 11.1% in the Rd arm (OR, 3.91; 95% CI, 2.62-5.84; P <.0001). Sustained MRD negativity that lasted for 12 months or more was also seen in more patients in the DRd arm than in the Rd arm, at rates of 18.8% vs 4.1% (P < .0001), and continued at 18 months or more at 16.8% vs 3.3% (P <.0001), respectively.
“What we’re learning is that being MRD negative is great, but it’s sustained MRD negativity over a duration of time, like 12 months, [that] is a much more powerful predictor of survival than…at single time points where MRD is negative,” explained Williams, a hematologist/oncologist and associate staff member at the Cleveland Clinic, to his group of physicians.
Additionally, the ORR favored patients receiving daratumumab compared with those who did not at a longer follow-up, at 92.9% (95% CI, 89.8%-95.3%) vs 81.6% (95% CI, 77.2%-85.4%), respectively (OR, 3.00; 95% CI, 1.85-4.86; P <.0001). A CR or better was seen in 51.1% of patients in the DRd arm compared with 30.1% in the Rd arm (OR, 2.44; 95% CI, 1.80-3.30; P < .0001), and stringent CR was also better in the study arm vs the control arm at 35.6% vs 15.7% (OR, 3.06; 95% CI, 2.14-4.38; P <.0001).
Williams also pointed out to his group that looking at sustained MRD negativity would be the key to other daratumumab-based treatments being effective in this patient population. This includes whether quadruplet therapies are to become a more preferred part of frontline treatment. According to Williams, the current nonrandomized MANHATTAN trial (NCT03290950) is looking at the use of daratumumab added to carfilzomib, lenalidomide, and dexamethasone, and initial results have shown promise for MRD negativity, but longer follow-ups are needed (From the Data7).

Moreover, patients with multiple myeloma require maintenance therapy for longer periods of time compared with patients with other cancers, so it is important for physicians to understand which triplet therapy will maintain patients’ disease the best. According to Garfall, it makes sense to continue the treatment as is until it is no longer tolerated, then readjust as needed, but he noted that many patients who continue treatment will be receiving daratumumab for longer periods of time.
Addressing Patient Frailty and Safety with the Addition of Daratumumab
Both groups agreed that it is important each patient be considered as an individual and their needs assessed that way. Some reasons patients are deemed ineligible for treatment are their age and comorbidities, meaning that many patients on a triplet regimen of DRd will be older and considered frail. But according to Williams, although patients who were not considered frail did better than those labeled as frail in the MAIA trial, frail patients still did better when they received daratumumab compared with other treatments.3
In a subgroup analysis of the MAIA trial, 396 patients were considered nonfrail, with 196 (53.3%) in the daratumumab arm vs 200 (54.2%) in the Rd arm. A total of 341 patients were designated as frail, with 172 (46.7%) in the DRd arm and 169 (45.8%) in the Rd arm. Median PFS, performed at a median follow-up of 36.4 months, was not reached (NR) but still maintained across the subgroups and favored patients receiving DRd in both the nonfrail (HR, 0.48; P <.0001) and frail groups (HR, 0.62; P = .003).
The nonfrail group was broken down further, with patients divided into fit, intermediate, and total nonfrail patients, compared with frail patients. Among these frailty subgroups, the most common reasons for treatment discontinuation were progression of disease and adverse events (AEs). The highest percentage of discontinuation of treatment due to AEs was in the frail group receiving Rd, at 18.9%, compared with the frail patients receiving DRd, at 9.9%.
Duration of treatment was longer in the total–nonfrail subgroup than in the frail group, but when researchers observed the difference between DRd and Rd, frail patients receiving DRd had a longer duration of treatment at a median of 31.1 months (range, 0.1-49.0) compared with 20.7 months (range, 0.03-41.9) among Rd patients. One of the considerations in this group was also the intensity with the use of lenalidomide; the median relative dose intensity of lenalidomide was higher in the DRd group for patients who ultimately discontinued lenalidomide, at 60.0% vs 88.4% in the Rd group.
In the Garfall group, Meher Burki, MD, a hematologist and medical oncologist at Penn Medicine Chester County Hospital, aired her concerns about lenalidomide tolerance, in particular with patients who have renal insufficiency. She explained that she typically starts with the lower dose and then dose adjusts up for these patients, a sentiment echoed by Garfall and other physicians who cautioned that comorbidities of this older patient population need to be considered.
In the MAIA trial, the most common grade 1 to 2 treatment emergent AEs (TEAEs) were diarrhea (57%), peripheral edema (40%), back pain (37%), and fatigue (36%), and the most common hematologic TEAEs were anemia (26%), thrombocytopenia (13%), and leukopenia (9%).2 Grade 3 neutropenia was seen in 37% of patients in the DRd arm and grade 4 neutropenia continued at 17%.
“It depends on their comorbidities. Certainly, I’m not going to give bortezomib to someone [who] has diabetic neuropathy; maybe if their [blood] counts are bad starting off, I would think about using daratumumab and bortezomib together. But largely, it’s about looking at the patient to decide which of the triplets we should use,” added Adam Waxman, MD, MS, an assistant professor of clinical medicine at the University of Pennsylvania, in the Garfall group.
References
1. Kumar SK, Moreau P, Bahlis NJ, et al. Daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in transplant-ineligible patients with newly diagnosed multiple myeloma (NDMM): Updated analysis of the phase 3 Maia study. Blood. 2022;140(suppl 1):10150-10153. doi:10.1182/blood-2022-163335
2. Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582-1596. doi:10.1016/S1470-2045(21)00466-6
3. Facon T, Cook G, Usmani SZ, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066-1077. doi:10.1038/s41375-021-01488-8
4. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 3.2023. https://bit.ly/41KZPMg
5. Facon T, San-Miguel J, Dimopoulos MA, et al. Treatment regimens for transplant-ineligible patients with newly diagnosed multiple myeloma: a systematic literature review and network meta-analysis. Adv Ther. 2022;39(5):1976-1992. doi:10.1007/s12325-022-02083-8
6. Facon T, Kuma S, Plesner T, et al; MAIA Trial Investigators. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380:2104-2115. doi:10.1056/NEJMoa1817249
7. Landgren O, Hultcrantz M, Diamond B, et al. Safety and effectiveness of weekly carfilzomib, lenalidomide, dexamethasone, and daratumumab combination therapy for patients with newly diagnosed multiple myeloma: the MANHATTAN nonrandomized clinical trial. JAMA Oncol. 2021;7(6):862-868. doi:10.1001/jamaoncol.2021.0611