A Look Back Prompts Memories of Initial Skepticism for Targeted Agents Shattered by Positive Efficacy Trials
After decades of use in clinical settings, targeted therapies now enjoy favored status in the cancer treatment landscape.
Howard A. “Skip” Burris III, MD
In 1992 when investigators began testing trastuzumab (Herceptin) in women with HER2-positive breast cancer, many observers predicted failure—and they kept on predicting failure throughout the trial process. Even after the FDA approved the drug in 1998, some observers scoffed at the modest survival benefit it had demonstrated to that point and predicted that targeted therapy would never be a major form of cancer treatment. “There was a great deal of skepticism about targeted monoclonal antibodies back in the 1990s. People didn’t believe that it would be possible to make antibodies that bound specifically to cancer cells or, if it was possible, that the antibodies would kill the cancer cells. It’s hard to imagine now, but those first trastuzumab trials I did in the mid-’90s were actually controversial,” said Howard A. “Skip” Burris III, MD, president of clinical operations and chief medical officer at Sarah Cannon in Nashville, Tennessee.
“It wasn’t until the subsequent trials that used trastuzumab in women with early-stage breast cancers, trials that showed a much bigger survival benefit than the trials in metastatic cancers, that everyone saw trastuzumab as a major breakthrough, and many people realized that targeted therapies might proliferate quickly,” he said.
There was, of course, reason for skepticism. A small number of successful targeted therapies existed longbefore trastuzumab, but none of them launched a new class of cancer treatments that successfully fought a wide range of tumor types.
Paul Bunn, MD

Indeed, physicians have known since the 1940s that iodine-131 therapy can selectively target thyroid cancer and that androgen deprivation therapy can selectively target prostate cancer. Hormone deprivation did find one other major use in the 1970s, when tamoxifen (Soltamox) was approved for hormone receptor–positive breastncancer, but neither iodine-131 nor any other elemental radioisotopes have become major treatment types.
Thus, many people doubted that the approval of the first 2 monoclonal antibodies, trastuzumab and rituximab (Rituxan), signified an explosion in new targeted therapies, and if not for rapid advances in genetics, proteomics, and molecular synthesis, the doubters may have been right.
Imatinib
But the 2001 approval of a third novel targeted therapy, imatinib mesylate (Gleevec), turned many skeptics into believers. Unlike trastuzumab and rituximab, imatinib is a small molecule drug, but it is a highly specific inhibitor of tyrosine kinase enzymes that are overactive in a number of cancers that are Philadelphia chromosome positive. It was also obvious, from the very first trials, that imatinib was an incredibly effective treatment, particularly for chronic myelogenous leukemia (CML). A phase 1 trial launched in 1998 reported that the drug caused CML to quickly disappear in the vast majority of patients with early-stage disease, and a 5-year follow-up found that 98% of patients were still in remission.1
Within a decade of trastuzumab’s initial approval, the FDA approved another 23 targeted cancer therapies. More than 80 targeted cancer therapies are now available to patients.2
“It’s been an amazingly rapid proliferation, and it’s happened because we’ve made huge technical advances on 2 distinct fronts,” Burris said. “When trastuzumab arrived, we were still working to sequence one human genome at a cost of many billions of dollars. Systematically analyzing tumors for druggable driver mutations seemed unrealistically expensive, but then, over the course of just a few years, the technology became so much faster and so much cheaper that it was entirely feasible. The other big advance has been on the chemistry side. Pharmaceutical and biotech companies have gotten much better at creating molecules that will bind to their intended targets. Better chemistry has allowed medications to bind to many targets like KRAS that were once considered undruggable.”
Monoclonal Antibodies
The first attempt to develop a targeted monoclonal antibody treatment for cancer dates back to 1980, when a team led by Lee Marshall Nadler, MD, treated a patient affected by non-Hodgkin lymphoma with the murine monoclonal antibody AB 89. The treatment, however, did not produce a significant clinical response.3
Several other research groups tested monoclonal antibodies against hematological cancers over the next decade, but none of them worked well enough for FDA approval. In fact, many of these murine formulations induced immune reactions that both created adverse effects and reduced therapeutic half-life.4
The first monoclonal antibody to demonstrate itself safe and effective enough for FDA approval was rituximab, which kills B cells by targeting the surface antigen CD20. The drug, which was first approved in 1997 for B-cell lymphoproliferative disorders, is currently indicated, alone or in various combinations, to treat forms of non-Hodgkin lymphoma and chronic lymphocytic leukemia.
Trastuzumab, which was approved a year later, is a genetically engineered, humanized monoclonal antibody that fights cancer 2 ways. First, it inhibits HER2, a glycoprotein receptor with tyrosine kinase activity that promotes breast cancer cell growth. Second, it induces cancer cell death via antibodydependent cell-mediated cytotoxicity.
Trastuzumab was developed at the University of California, Los Angeles (UCLA) by a team led by Dennis Slamon, MD, PhD. Slamon identified the HER2-positive subtype of breast cancer in the early 1980s, demonstrated that this subtype was particularly deadly and aggressive, and hypothesized that a HER2 inhibitor would extend life. The team spent several years developing drug candidates, and the very first human trials were conducted at UCLA in 1990.
Trastuzumab has since shown significant—and often dramatic—benefit when used in a variety of ways against HER2-positive breast cancer. For example, a 2005 trial of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer found that after a median follow-up of one year, 1693 women assigned to the observation group had nearly twice as many events (recurrence, contralateral breast cancer, second non–breast malignant disease, or death) as the 1694 women assigned to the trastuzumab group.5
In the early 1990s, women with the HER2-positive subtype had an average life expectancy after diagnosis of 3 to 5 years. Today, an estimated 3 million patients have an average life expectancy of 7 to 10 years, thanks largely to receiving trastuzumab.6
Gefitinib and Erlotinib
The next targeted therapy to reach patients was gefitinib (Iressa), an EGFR tyrosine kinase inhibitor (TKI) approved in 2003 for the treatment of non–small cell lung cancer (NSCLC). Studies published the following year showed that a particular subset of patients with EGFR mutation– positive NSCLC were far more likely than others to respond to gefitinib7 and a second EGFR TKI approved in 2004, erlotinib (Tarceva).8
The phase 3 trial that led to erlotinib’s approval found that only 8.9% of patients responded to the drug and that it increased median overall survival (OS) by just 2 months to 6.7 months,9 but the subgroup analysis found traits ranging from female sex to never smoking to EGFR expression that predicted response.
Later research demonstrated that EGFR TKIs greatly increase survival in patients with EGFR-positive NSCLC. Combination chemotherapy in advanced NSCLC typically results in a median OS of 8 to 12 months and a median progression-free survival (PFS) of 5 to 6 months.10 Among 137 patients with EGFR-mutant metastatic lung adenocarcinoma treated with erlotinib or gefitinib at Dana-Farber Cancer Institute between 2002 and 2009, however, median PFS and OS were 12.1 months and 30.9 months, respectively. Twenty patients (14.6%) were 5-year survivors.11
“Even though targeted therapies had already proven themselves effective against other tumor types, especially imatinib against CML, a lot of people were still skeptical that they would work against lung cancer,” said Paul Bunn, MD, distinguished professor of medicine-medical oncology and the James Dudley Chair in Cancer Research at the University of Colorado School of Medicine in Aurora, Colorado. “But the skeptics were quickly silenced. The trial results were pretty convincing.”
Antiangiogenics
Shortly after the FDA approved erlotinib, it also approved bevacizumab (Avastin), the first example of another novel form of targeted therapies called antiangiogenics, which are designed to deprive tumors of blood.
Many targeted therapies were conceived long before they were approved, including antiangiogenics. The idea of fighting cancer by starving tumors of blood first occurred to Judah Folkman, MD, in 1963, when he and Fred Becker, MD, were comparing possible substitutes for blood transfusions. During those experiments, the 2 young doctors injected adult mouse melanoma cells into isolated, perfused thyroid glands taken from dogs and noticed that while tumors did form, they never developed blood vessels or grew beyond 2 mm in diameter.12
Other investigators had already made similar observations, but Folkman’s efforts to understand his findings led him to complete a few more experiments and then to hypothesize, via a 1971 piece in the New England Journal of Medicine, 13 several very new ideas:
- Tumors cannot grow dangerously large unless they develop vascular networks.
- Tumors cannot build their own vascular networks, so they must trick their hosts into building such networks through angiogenesis.
- An angiogenesis inhibitor could therefore treat cancer effectively.
The paper drew such negative response that it quickly made Folkman a pariah.
The approval of bevacizumab in 2004 was a triumph for Folkman, but because he died in 2008, he did not live long enough to see how broadly the use of antiangiogenics would expand. Bevacizumab alone is now approved as monotherapy or in combination with other medications to treat 7 different tumor types,14 and more than a dozen other angiogenesisinhibiting medications have subsequently been approved as cancer treatments.15
Many of them work by binding to VEGF, preventing it from activating the VEGF receptor, and blocking the formation of new blood vessels. The first 2 approved drugs that targeted this pathway were sorafenib (Nexavar), which was approved in 2005, and sunitinib malate (Sutent), which was approved in 2006.16,17
Oddly, an antiangiogenic drug had already been approved for use in most of the world (just not the United States) slightly before Folkman first devised the idea in 1963, but it was approved to treat sleeplessness and morning sickness rather than cancer. That drug, of course, was thalidomide (Synovir, Thalomid), which was later approved to treat multiple myeloma, many decades after the discovery that it causes birth defects.
PARP Inhibitors
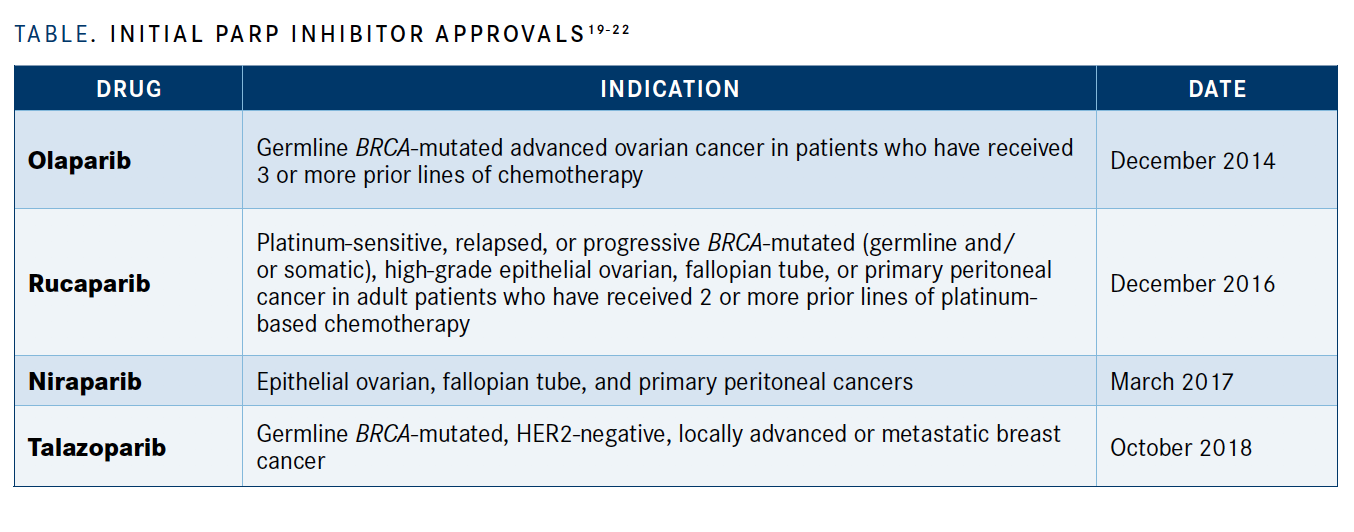
Molecules that inhibit PARPs, which play a major role in DNA damage repair, were another form of targeted therapy that existed long before FDA approval to treat cancer. PARP-inhibiting substances, such as nicotinamide, have been used for various indications over the decades. In the 1970s, some thought these substances might prime patients for better response to chemotherapy or radiation.18
The FDA, however, did not support a PARP inhibitor for cancer treatment until 2014, when it approved olaparib (Lynparza) for advanced ovarian cancer with a deleterious or suspected deleterious germline BRCA mutation.19 It has since approved more PARP inhibitors, including\ rucaparib (Rubraca), niraparib (Zejula), and talazoparib (Talzenna) across a wide variety of tumors, including metastatic prostate cancer and certain subtypes of breast cancer (TABLE19-22).20-22
Immunotherapies
Immunotherapies are another type of targeted cancer treatment that were hypothesized many decades before they were realized. A German physician working in the mid-19th century noticed that tumors tended to regress when cancer patients were infected by erysipelas, so in 1868, he intentionally infected a patient with erysipelas to treat their cancer.23
In the 19th century, the American doctor William Coley began directly injecting tumors with mixtures of live and inactivated Streptococcus pyogenes and Serratia marcescens. He reported more than 1000 regressions or cures over the course of his career, but he was poorly esteemed among researchers of the day and his findings were largely ignored.24
The work that laid the foundation for today’s immunotherapies took place in 1982, nearly a century after Coley’s first efforts at immunotherapy, when James Allison, PhD, and colleagues identified and described tumor-specific antigens in a mouse model of T-cell lymphoma. 25 A year later, they described the first T-cell antigen receptor.
The first immune checkpoint molecule was discovered in 1987 and named CTLA-4. Just one year later, the first CTLA-4–blocking antibody was discovered, and in 2011, the first CTLA-4–blocking drug, ipilimumab (Yervoy), was approved by the FDA.26
Look to the Future
“The sheer number of targeted therapies that have been approved in the [past] 20 years is amazing, even if you’re just looking at my specialty of lung cancer,” said Bunn, who is a past president of the American Society of Clinical Oncology. “And thanks to those treatments, along with earlier diagnosis, survival rates have improved considerably, from roughly 4 to 6 months untreated to a year with chemotherapy to 5 years or more with TKIs. But there’s still work to be done because neither the TKIs nor the immunotherapies, or anything else, tends to produce complete responses or absolute cures. We need to find things we can pair with TKIs to kill the cancer cells they don’t, and there are a large number of combinations that are currently being investigated.”
Burris is also excited to see so many new targeted treatments—and combinations of targeted treatments—in trials right now. He is particularly optimistic about antibody-drug conjugates, therapies that combine antibodies that bind to tumors with a second agent that attacks the tumor. The most successful such product may be ado-trastuzumab emtansine (Kadcyla), which combines trastuzumab with a cytotoxic drug that’s released after the molecule binds to the cancer.
“When you see what big pharmaceutical companies are paying to acquire the makers of antibody- drug conjugates, you get a good sense of how promising the technology is,” Burris said. “The technology for binding the antibody and the cytotoxic agent, for getting the antibody to bind with the tumor, and for releasing the cytotoxic agent at the right time all keep improving. This is a promising strategy for attacking a wide range of tumors that don’t have a druggable driver mutation.”
References:
1. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J. Med. 2006;355(23):2408-2417. doi: 10.1056/NEJMoa063867
2. Targeted therapies: overview of targeted therapies for cancer. My Cancer Genome. Updated May 27, 2019. Accessed November 2, 2020. https://bit.ly/385txmr
3. Nadler LM, Stashenko P, Hardy R, et al. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980;40(9):3147-3154.
4. Dillman RO, Beauregard JC, Halpern SE, Clutter M. Toxicities and side effects associated with intravenous infusions of murine monoclonal antibodies antibodies. J Biol Response Mod. 1986;5(1):73-84.
5. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al; Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659-1672. doi:10.1056/NEJMoa052306
6. UCLA oncologist Dennis Slamon wins 2019 Lasker Award for clinical medical research. UCLA newsroom. September 9, 2019. Accessed November 2, 2020. https://bit.ly/2TKzvkt
7. Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497-1500. doi:10.1126/science.1099314
8. Tsao M-S, Sakurada A, Cutz J-C, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133-144. doi:10.1056/NEJMoa050736
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated nonsmall-cell lung cancer. N Engl J Med. 2005;353(2):123-132. doi:10.1056/NEJMoa050753
10. Schiller JH, Harrington D, Belani CP, et al; Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced nonsmall- cell lung cancer. N Engl J Med. 2002;346(2):92-98. doi:10.1056/NEJMoa011954
11. Lin JJ, Cardarella S, Lydon CA, et al. Five-Year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol. 2016;11(4):556-565. doi:10.1016/j.jtho.2015.12.103
12. Folkman J, Long DM Jr, Becker FF. Growth and metastasis of tumor in organ culture. Cancer. 1963;16:453-467. doi:10.1002/1097-0142(196304)16:4<453::aid-cncr2820160407>3.0.co;2-y
13. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182-1186. doi:10.1056/NEJM197111182852108
14. Bevacizumab. National Cancer Institute. October 5, 2006. Updated September 10, 2020. Accessed November 2, 2020. https://bit.ly/2TKcNcn
15. Angiogenesis inhibitors. National Cancer Institute. Updated April 2, 2018. Accessed November 2, 2020. https://bit.ly/325ldQ0
16. Nexavar. Prescribing information. Bayer HealthCare Pharmaceuticals Inc; 2010. Accessed November 2, 2020. https://bit.ly/360eQid
17. Sutent. Prescribing information. Pfizer Inc; 2011. Accessed November 2, 2020. https://bit.ly/2I0jzbr
18. Clark JB, Ferris GM, Pinder S. Inhibition of nuclear NAD nucleosidase and poly ADP-ribose polymerase activity from rat liver by nicotinamide and 5’-methyl nicotinamide. Biochim Biophys Acta. 1971;238(1):82-85. doi:10.1016/0005-2787(71)90012-8
19. FDA approves olaparib tablets for maintenance treatment in ovarian cancer. FDA. Updated August 17, 2017. Accessed November 3, 2020. https://bit.ly/324l5Ah
20. FDA grants accelerated approval to new treatment for advanced ovarian cancer. News release. FDA. December 19, 2016. Accessed November 3, 2020. https://bit.ly/34Pzsu7
21. Niraparib (Zejula). FDA. Updated May 30, 2017. Accessed November 2, 2020. https://bit.ly/36bQT7J
22. FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer. FDA. Updated December 14, 2018. Accessed November 2, 2020. https://bit.ly/3jUsp7r
23. Busch W. Aus der Sitzung der medicinischen Section vom 13 November 1867. Berlin Klin Wochenschr. 1868;5:137. (Ger).
24. Dobosz P, Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front Immunol. 2019;10:2965. doi:10.3389/fimmu.2019.02965
25. Allison JP, McIntyre BW, Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol. 1982;129(5):2293-2300.
26. Yervoy. Prescribing information. Bristol Myers Squibb; 2018. Accessed November 2, 2020. https://bit.ly/2HQsQT7

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More