Worden Discusses Use of Hedgehog Pathway Inhibitors in Treating Basal Cell Carcinoma
During a Targeted Oncology case-based roundtable event, Francis Paul Worden, MD, discussed treatment options for a patient with high-risk basal cell carcinoma.

Francis Paul Worden, MD
Clinical Professor
University of Michigan Rogel Cancer Center
Ann Arbor, Michigan

Targeted OncologyTM: How is BCC stratified?
WORDEN: The NCCN guidelines divide BCC into low and high risk, [which is based on] location and size. If it is on the trunk and extremities, and [the tumor] is less than 2 cm, it is of low risk. Lesions, such as in this patient, which was a larger BCC greater than 2 cm, are high risk. Mask areas of the face, such as the eyelids, eyebrows, and areas of the neck and forehead, are all linked to high-risk features. Lesions with poorly defined borders are also high risk.1
Oftentimes, high-risk BCC is recurrent, so they’re treated surgically and come back, or they’re treated with radiation and come back and immunosuppression is common. Patients with CLL [chronic lymphocytic leukemia] and patients on immunosuppressive agents for kidney transplant, for example, are at a higher risk of developing these cancers, [as well as] those who had prior radiation therapy. The high-risk subtype has an aggressive growth pattern when observed histologically and, in general, there’s perineural involvement.
Based on this patient’s high risk for recurrence, what are the treatment options?
With almost any other head and neck cancer, we would start with a surgical option, then consider radiation therapy. [In general], systemic therapy is saved for those who are not candidates for surgery or radiation. A lot of these patients will have had recurrent cancers, so they keep coming back after they’ve already been radiated or surgically excised. I think the biggest challenge is, like in this case, where the patient has 1 lesion, so aggressive local measures could be taken into consideration. Oftentimes, such patients will present with multiple lesions, then it becomes trickier. They can’t all be surgically excised, and radiation would be a difficult approach, given they mostly occur on sun-exposed surfaces, such as the head and neck. A wide variety of cutaneous squamous cell carcinoma [SCC] and BCC occur in the head and neck.
After you’ve entertained local measures, the systemic measures come into play. I do not see much of a difference between vismodegib [Erivedge] and sonidegib [Odomzo]. Vismodegib was first approved, and that’s usually my go-to. In the context of surgery—and perhaps radiation therapy—we recently published an article in The Oncologist about the treatment of cutaneous BCC involving the eyelid, where we treated patients with vismodegib and were able to eliminate the disease in a number of them.2 Some were limited by adverse effects [AEs], but we were able to downsize the tumor, so 100% of the patients had organ preservation and none of them lost their eye. So you could go back to local measures once you’ve considered systemic measures.
Would you start with cemiplimab (Libtayo) first?
In general, we would start with the hedgehog pathway inhibitor [HPI] first, and if they are not a good candidate or have intolerance, perhaps, then we go to cemiplimab. There are patients who have diffuse BCC formations, [such as] in Gorlin syndrome, and patients who have multiple tumors who use the HPIs with a great degree of success.1
Was there any systemic imaging for this patient with locally advanced disease? Is a PET scan or total-body CT scan done?
Generally, you can see metastatic disease with scans. For patients [who] have disease primarily located on the head and neck without extensive adenopathy or bulky disease, we don’t routinely get CT scans of the chest, which is the first site of metastatic deposit. If someone has axillary involvement, then sure, we will do a chest CT scan. With melanoma, we will do it too, but not necessarily with the BCC. It’s not unreasonable for us to get that as a baseline, but I wouldn’t keep doing it again and again, because the response is going to be based on clinical examination.
So yes, I think a chest CT scan is fine, and I wouldn’t necessarily get a PET scan unless you have different findings on different CT scans. If you have systemic disease involving more than just the head and neck, it may be reasonable to consider either a PET scan or a CT scan involving the abdomen and pelvis, but I wouldn’t necessarily do them because those are sun-exposed surfaces. I wouldn’t go after imaging studies in every single patient. It’s on a case-by-case basis.
Can you combine vismodegib with radiation therapy? Do patients get big scars or require big grafts?
There [are] no published data on that, but I think radiation oncologists don’t favor it, [in general]. As a medical oncologist, to be honest, most of the time, when we see these patients, they’ve been previously treated with radiation. There were data for cutaneous SCC, where they had weekly carboplatin plus radiation vs radiation alone, and the radiation-alone arm was the winner, but there was no benefit of adding chemotherapy.3 For most patients with cutaneous SCC, we don’t treat them with up-front chemotherapy plus radiation therapy. Radiation is sufficient and usually is an adjuvant to surgical resection unless surgery is contraindicated for reasons of cosmesis.
How does the hedgehog pathway work?
In the hedgehog pathway, the PTCH1 protein is blocked, which turns on the target of these genes in the nucleus, then it turns off the pathways that induce apoptosis and other mechanisms to produce cell death.4
What is your experience with using HPIs?
The FDA-approved HPI drugs are sonidegib and vismodegib, and they’re administered orally. For drug interactions, the CYP3A4 inhibitors or inducers can be an issue.5-7 [For those who haven’t used these medications], the point here is that the AE profiles are very similar, so it’s not like you can get away with an AE by using a different one, like what we run into with pancreatitis in CLL agents.
Muscle spasms and arthralgia can be limiting, but nausea and vomiting are not so bad, so we usually prescribe prochlorperazine [compazine] for patients. As with a lot of targeted inhibitors, patients eventually develop long-term fatigue, which can be very limiting for patients, where they are unable to get out of bed, for example. Dysgeusia leads to weight loss. There is also an increase in creatinine kinase. These can be difficult for a lot of patients.8-10 In the [VISORB trial (NCT02436408)],2 we treated patients until they could not take it anymore and had to come off treatment.
An interesting thing to point out is these drugs are all or nothing and not like other cancer treatments, where you can stop after grade 1 toxicities, then start at a lower dose. In studies where they tried that, the agents stopped working. So if you can’t tolerate it, you basically come off therapy.8-10
How do you manage toxicities of HPIs?
Amlodipine [Norvasc] [and L-carnitine (Carnitor)] has had some success. Patients who have liver failure and are candidates for transplant or not, get muscle spasms due to the rise in ammonia levels. A study done with L-carnitine—300 mg vs 400 mg 3 times daily—showed that in patients on 400 mg, the muscle spasms improved.
Based on that data, and with great success, I have used L-carnitine. You can have patients buy it at the health food store. A lot of them will say if they have a bit of a breakthrough. I’ve even increased the dose to 4 times daily, and it makes a huge difference. That is one thing I’ve found to be useful—and it’s a natural amino acid, so it’s not an issue. Some dermatologists use muscle relaxants, [such as] Flexeril [cyclobenzaprine] 5 mg to 10 mg at bedtime.8-10
Do you start L-carnitine prophylactically or after they get the muscle pains?
I don’t know what the exact right way to do it is in general, but I have started it right away, and I tell patients they’re going to develop these cramps, and it will help—plus it is innocuous. The insurance will cover it once in a great while if you prescribe it. So yes, I just start it right away, but the important point to remember is the dosage, which is 400 mg 3 times daily. If you use less than that, it won’t work.10
What are the toxicities of L-carnitine?
I have not had any, including any increase in serum creatinine. Patients don’t get an upset stomach. They tolerate it quite well. What they will say, especially if we start it after the patients have developed cramping or they forget to take it, is that they noticed a huge difference. So that is a trick of the trade that is probably the best I’ve found to help manage muscle cramps, which I think is the most dose-limiting AE.
What are the response rates for HPIs?
The overall response rate [ORR] for vismodegib in locally advanced BCC [laBCC] is [approximately] 43%, and for metastatic BCC, as we would expect, is a little lower [at 30%]. Sonidegib has only been tested and approved in the population with locally advanced BCC, and [the ORR is 44%]—very similar to vismodegib.11 In this case, the patient was started on vismodegib 150 mg daily, so we assume he was not a candidate for surgery or radiation therapy up front.
[In the phase 2 ERIVANCE] study [NCT00833417] [of vismodegib] in patients with [advanced] BCC, they divided patients into those with laBCC and metastatic BCC [mBCC]. Some of these patients presented with lesions on the head and neck or the face, like that of the patient in our case.11
Based on the data, patients with mBCC were less likely to obtain better responses than the patients with laBCC. This could be because of tumor burden or location. Nonetheless, both groups still had good responses and even stabilization of disease. The duration of response was about 8 months for both cohorts.11
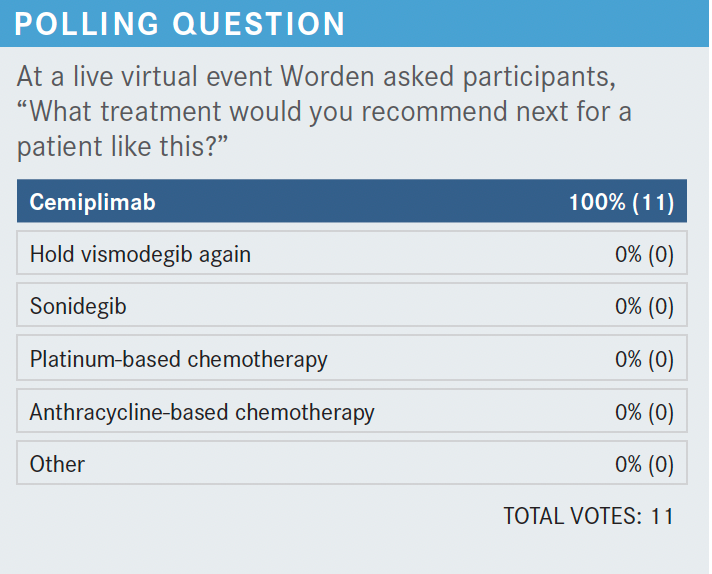
What are other expected toxicities of HPIs? How do you manage them?
We talked a bit about using calcium channel blockers. Some people use cold packs or quinine for restless leg syndrome if they’re having a difficult time with cramping, especially at night.
Most of the AEs were lower grades, including muscle spasms, arthralgia, [and] dysgeusia, where patients complain of a metallic taste in their mouths. Alopecia is something you should warn patients about up front. A few have nausea and prochlorperazine works. I don’t think you need to go to a serotonin reuptake inhibitor, [such as] Zofran [ondansetron] or Kytril [granisetron], but they could be considered. Constipation can occur, and a little of prochlorperazine seems to control the symptoms quite well.8-10
Fatigue is the biggest issue some patients run into, and it is not [just] arthralgia but long-term fatigue. The decreased appetite leading to decreased weight also compounds the fatigue.8

What would you recommend next?
The phase 2 REGN2810 study [NCT03132636] shows the benefit of cemiplimab in the population with mBCC vs laBCC. In the patient characteristics, previous exposure to HPIs was prevalent, [with exposure to vismodegib at 94%, sonidegib at 17%, and both at 11%].12
The responses occurred relatively quickly, and some of the patients were still on treatments. The ORR was 31% with 6% of patients getting complete response, which is great. The disease control rate was 80%, and the durable disease control rate was [approximately] 60%.12 That’s the beauty behind these drugs, and that’s why they are great alternatives that need to be explored more in combination with other agents, [perhaps]. The progression-free survival rates at 6 and 12 months were quite good, [with 76% at 6 months and 57% at 12 months].12
Pembrolizumab [Keytruda] [alone or in combination with vismodegib] has also been studied in advanced BCC.13 The ORR was a little lower than in single-agent pembrolizumab. This hasn’t been approved by the FDA. [However], there is an advantage with adding the checkpoint inhibitor, so I don’t think it’s unreasonable to consider combining the 2 drugs.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Basal cell skin cancer, version 2.2021. Accessed December 27, 2021. https://bit.ly/3mxK4q0
2. Kahana A, Unsworth SP, Andrews CA, et al. Vismodegib for preservation of visual function in patients with advanced periocular basal cell carcinoma: the VISORB trial. Oncologist. 2021;26(7):e1240-e1249. doi:10.1002/onco.13820
3. Porceddu SV, Bressel M, Poulsen MG, et al. Postoperative concurrent chemoradiotherapy versus postoperative radiotherapy in high-risk cutaneous squamous cell carcinoma of the head and neck: the randomized phase III TROG 05.01 trial. J Clin Oncol. 2018;36(13):1275-1283. doi:10.1200/JCO.2017.77.0941
4. Low JA, de Sauvage FJ. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol. 2010;28(36):5321-5326. doi:10.1200/JCO.2010.27.9943
5. Harris L. Basal cell carcinoma: a pharmacist’s guide. US Pharmacist. August 19, 2019. Accessed December 27, 2021. https://bit.ly/3xnc8Pd
6. Erivedge. Prescribing information. Genentech USA, Inc; 2020. Accessed December 27, 2021. https://bit.ly/3jlFDMw
7. Odomzo. Prescribing information. Sun Pharma Global FZE; 2019. Accessed December 27, 2021. https://bit.ly/3xkqGz1
8. Lacouture ME, Dréno B, Ascierto PA, et al. Characterization and management of Hedgehog pathway inhibitor-related adverse events in patients with advanced basal cell carcinoma. Oncologist. 2016;21(10):1218-1229. doi:10.1634/ theoncologist.2016-0186
9. Yang X, Dinehart S. Practical tips for managing Hedgehog pathway inhibitor side effects. Practical Dermatology. January 2017. Accessed December 27, 2021. https://bit.ly/3A8YNf8
10. Dinehart MS, McMurray S, Dinehart SM, Lebwohl M. L-carnitine reduces muscle cramps in patients taking vismodegib. Journal of Cutaneous Medicine and Surgery. Accessed December 27, 2021. https://bit.ly/3ltbmhu
11. Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171-2179. doi:10.1056/NEJMoa1113713
12. Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):848-857. doi:10.1016/ S1470-2045(21)00126-1
13. Chang ALS, Tran DC, Cannon JGD, et al. Pembrolizumab for advanced basal cell carcinoma: an investigator-initiated, proof-of-concept study. J Am Acad Dermatol. 2019;80(2):564-566. doi:10.1016/j.jaad.2018.08.017

















