Vaishampayan Reviews Frontline IO/TKI Regimens for Favorable-Risk ccRCC
During a Targeted Oncology case-based roundtable event, Ulka N. Vaishampayan, MBBS, discussed the frontline combination regimens available for a patient with favorable risk clear cell renal cell carcinoma.
Ulka N. Vaishampayan, MBBS
Clinical Professor of Internal Medicine
Director of the Phase I Program
Rogel Cancer Center
University of Michigan
Ann Arbor, MI

Targeted OncologyTM: What regimens do the National Comprehensive Cancer Network (NCCN) guidelines recommend for patients with favorable-risk vs poor- or intermediate-risk relapsed or stage IV kidney cancer? Do you have a preference among these regimens?
VAISHAMPAYAN: I’m not sure the guidelines are terribly helpful in this case because for favorable-risk disease, these are the 3 regimens: axitinib [Inlyta] plus pembrolizumab [Keytruda]; cabozantinib [Cabometyx] plus nivolumab [Opdivo]; and lenvatinib [Lenvima] plus pembrolizumab. Subgroup analyses of favorable-risk patients showed a benefit for each of these regimens, so that’s why there is a category 1 recommendation for each. For intermediate- and poor-risk disease, all 3 of these regimens are recommended, as are ipilimumab [Yervoy] plus nivolumab and the single-agent tyrosine kinase inhibitor [TKI] cabozantinib, based on [data from] a phase 2 randomized trial. Axitinib plus avelumab [Bavencio] was also a regimen that had initially shown efficacy with better response rates and better progression-free survival [PFS] but did not quite show overall survival [OS] benefit, and that’s why it is listed among “other recommended regimens.”1
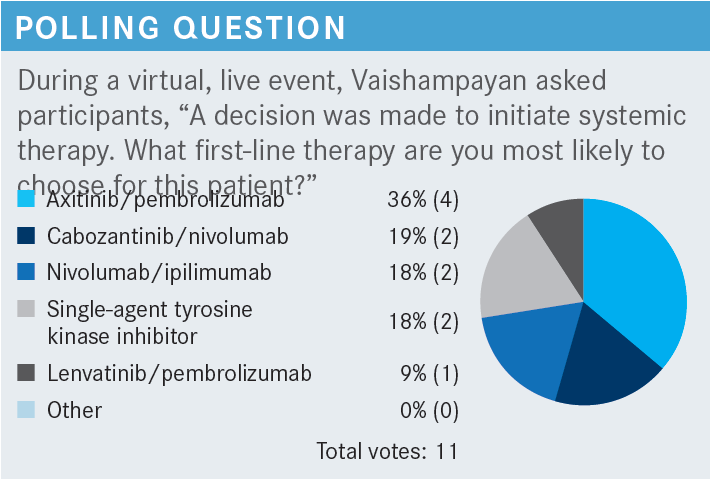
Typically, for favorable risk, I have mixed feelings about whether to have that balanced risk-benefit discussion and how to try to do as little as possible. But definitely in a young patient like this, where you’re beginning to see lung and mediastinal lymph node metastases, I would probably go with a VEGF-TKI and [immuno-oncology; IO] regimen.
As far as the VEGF-TKIs, there’s no real difference between each of these; for the most part, I think as long as you can manage the toxicities well and you give the patient a heads-up about the toxicities and have them tell you when they have these toxicities, I think you couldn’t go wrong. I typically lean toward axitinib/pembrolizumab. Cabozantinib/nivolumab and lenvatinib/pembrolizumab are more recent. A little more follow-up time has occurred for ipilimumab/nivolumab and axitinib/pembrolizumab, and clearly only because those were the studies that started first. The CheckMate 9ER [NCT03141177] and CLEAR [NCT02811861] studies are more recent. That’s the only reason why I may favor older regimens.

What data support the use of cabozantinib plus nivolumab to treat patients with untreated advanced RCC?
The CheckMate 9ER study led to the FDA approval of cabozantinib/nivolumab,2 which was compared with sunitinib [Sutent], our default standard at the time. All the patients had to have a clear cell component. Any International Metastatic RCC Database Consortium [IMDC] risk group was eligible. Tumor PD-L1 expression [was another stratification parameter]. Interestingly, in almost every study, there is stratification by PD-L1 expression, and for kidney cancer, the PD-L1 expression did not seem to make an impact on whether patients benefited or not. So it was not a good predictive marker of efficacy. The primary end point in this study was PFS.3
After 33 months’ median follow-up, clearly there was a statistically significant survival difference. Right in the first 2 or 3 months, the PFS [Kaplan-Meier] curves separated, and they have stayed separate. At the 2-year mark, the landmark analysis showed that [20.9%] of the patients in the control arm had not progressed, compared with 39.5% (almost double) in the cabozantinib/nivolumab arm. Median PFS was doubled from 8.3 months [95% CI, 7.0-9.7] to 16.6 months [95% CI, 12.8-19.8], respectively [HR, 0.56; 95% CI, 0.46-0.68].4
In terms of OS, [70.3%] of patients [in the cabozantinib/nivolumab arm] were alive at 2 years, remarkably, compared [with 60.3%] with sunitinib therapy. The objective response rate [ORR] was doubled from 28.4% with sunitinib to 55.7% with the combination. The complete response [CR] rate was also doubled from [5.2%] with sunitinib to [12.4%] with the combination.4
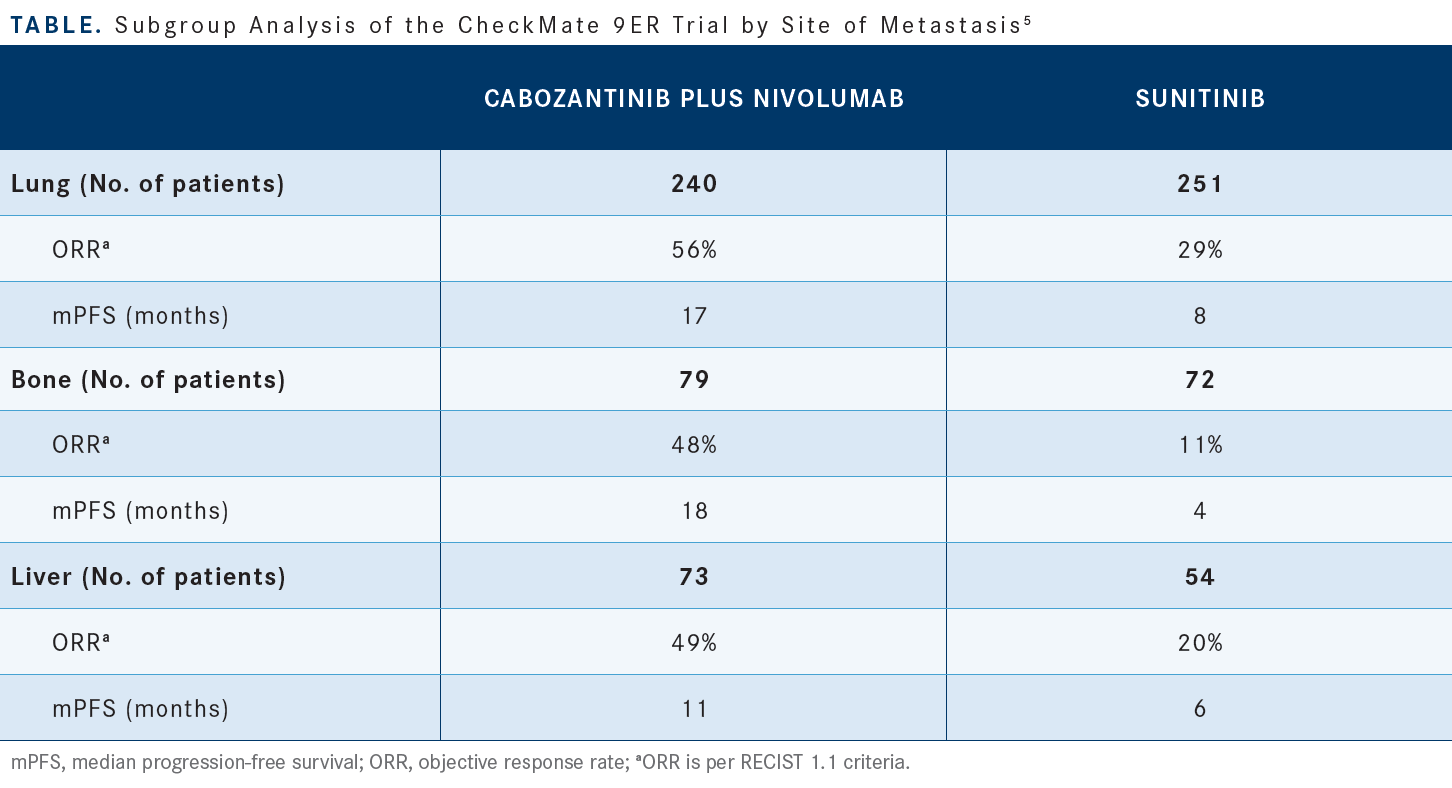
Then each of the subgroups, defined by sites of metastases, by IMDC risk, [either favorable, intermediate, or poor], and extent of tumor burden at baseline, favored cabozantinib/nivolumab. For all subgroups defined by sites of metastases, [like the lung, liver, and bone], both PFS and OS significantly favored cabozantinib/nivolumab compared with sunitinib [Table5]. The group with liver metastases had a much worse prognosis [than the other groups, but] there wasn’t a big difference in PFS or OS.5 These are subset analyses, but they yielded very encouraging data showing that the efficacy of the combination is there, regardless of these different sites of metastases.
The incidence of severe adverse events [AEs] was relatively small, but AEs of grade 3 or higher were seen in 65.0% of the patients who received cabozantinib/nivolumab. As you know, most of the VEGF-TKIs have a class effect of hypertension, diarrhea, skin rash, and fatigue, [which were observed among patients in both arms of this study].4 Usually when you stop the medication, most of those AEs do turn around. Depending on how severe the AE was and how long it took for the patient to get better, you can decide if you need to reduce the dose.
The starting dose of cabozantinib in this study was 40 mg daily. The dose reduction was down to 20 mg daily or 20 mg every other day, if the patient had recurrent toxicities.4 The way to differentiate between immune-related colitis and cabozantinib-induced diarrhea, [for example, is to] stop both [drugs], and if the symptomatic therapy works, then most likely it’s VEGF-TKI–related diarrhea. If, however, it is not getting better and it’s time to start steroids, consider it an immune-related AE.
What data support the use of axitinib plus pembrolizumab in patients with newly diagnosed or recurrent stage IV ccRCC?
[The KEYNOTE-426 trial; NCT02853331] looked at axitinib/pembrolizumab [in this population]. Both PFS and OS data were reported after a median 42-month [follow-up]. This study was started earlier than CheckMate 9ER. In KEYNOTE-426, the standard doses of axitinib, 5 mg twice daily, and sunitinib [50 mg oral daily] were given. [Compared with the CheckMate 9ER trial, this study had] similar eligibility criteria and stratification factors—IMDC risk group and geographic region. This study did not stratify by PD-L1 status.6
KEYNOTE-426 had a dual primary end point of PFS and OS. The median OS in the intention-to-treat population, at [42.8] months’ median follow-up, [was 45.7 months (95% CI, 43.6-not reached [NR]) vs 40.1 months (95% CI, 34.3-44.2) in the experimental and control arms, respectively]. The OS difference at 30 months was 67% vs 59%, respectively, and there were long-term durable remissions with axitinib/pembrolizumab [HR, 0.73; 95% CI, 0.60-0.88; P < .001]. Median PFS was 15.7 months [95% CI, 13.6-20.2] with axitinib/pembrolizumab and [11.1] months [95% CI, 8.9-12.5] with sunitinib [HR, 0.68; 95% CI, 0.58-0.80; P < .0001]. The ORR was [60.4%] with axitinib/pembrolizumab and 39.6% with sunitinib.7,8
The AEs were similar [to those seen in the CheckMate 9ER study, including] axitinib-related hypertension, diarrhea, and skin rash. Pembrolizumab had the similar immune-related AEs of colitis, pneumonitis, transaminitis, and so on.7,8
Are there data showing efficacy of the cabozantinib, ipilimumab, and nivolumab triplet for the treatment of advanced RCC?
[Data have been presented recently] about the triplet therapy of cabozantinib/ipilimumab/nivolumab vs ipilimumab/nivolumab [COSMIC-313; NCT03937219]. They showed that PFS was improved with the triplet regimen vs ipilimumab/nivolumab alone. This is the first study that did not have sunitinib as the control arm; it used ipilimumab/nivolumab as the control arm. [It was reported] that PFS was improved with the triplet [NR (95% CI, 14.0- not estimable) vs 11.3 months (95% CI, 7.7-18.2) with ipilimumab/nivolumab; (HR, 0.73; 95% CI, 0.57-0.94; P = .013)] but [there has been] no OS benefit so far. Of course, these data are not mature enough to report OS.9
What data support the use of first-line lenvatinib plus pembrolizumab in patients with advanced ccRCC?
[Those data come from] the CLEAR study, which is the more recent study. This was a 3-arm study; the arms were lenvatinib/pembrolizumab, lenvatinib plus everolimus [Afinitor], and sunitinib. The primary end point was PFS, and secondary end points were OS, ORR, [safety, and quality of life].10
Both combinations improved PFS compared with sunitinib, but lenvatinib/pembrolizumab clearly was the winner, showing remarkable PFS improvement with a median PFS of [23.9] months [(95% CI, 20.8-27.7). PFS was 14.7 months with lenvatinib/everolimus (95% CI, 11.1-16.7) and 9.2 months with sunitinib (95% CI, 6.0-11.0).]10
These are relatively less mature data, but the OS results are starting to come together. So far, it is looking like OS favors the lenvatinib/pembrolizumab combination10 [for lenvatinib/pembrolizumab vs sunitinib (HR, 0.72; 95% CI, 0.55-0.93)].11 The ORR also was the highest with the combination of lenvatinib/pembrolizumab, [at 71.0%], vs [53.5% with lenvatinib/everolimus] and [36.1%] with sunitinib. The CR rates were [16.1%] with lenvatinib/pembrolizumab, 9.8% with lenvatinib/everolimus, and 4.2% with sunitinib.10
The AE profile [of lenvatinib/pembrolizumab] was not terribly different from those of the other 2 VEGF-TKI and IO combinations. Grade 3 or higher AEs [occurred in 82.4% of patients in the lenvatinib/pembrolizumab arm]. The incidence of severe diarrhea and hypertension was slightly higher in the lenvatinib/pembrolizumab arm, but each of these agents had similar class effects.10
In summary, how do these combinations compare in terms of efficacy?
The CLEAR trial [of lenvatinib/pembrolizumab] showed the highest ORR [71%]. For PFS and OS, especially when an immune regimen is involved, you want to look at the long-term remission rate; that is similar, so far, for each of these agents [in the 80% to 90% range for 12-month OS and in the 70% to 75% range for 24-month OS]. [These results] look promising and similar across each of these therapies.3-5,7,8,10-12
Treatment-related AEs [TRAEs] also were not that much different [across each of these therapies, with approximately] 90% of patients affected by TRAEs of any grade. Almost every patient had some AEs. TRAEs of grade 3 or higher affected 50% to 60% [of patients receiving each regimen]. For the lenvatinib/pembrolizumab combination, approximately 72% were affected by grade 3 or higher TRAEs, slightly worse than the other combinations. Approximately 20% to 30% of the patients [in the KEYNOTE-426 and CheckMate 9ER trials needed] steroids; for the CLEAR trial, steroid use has not been reported.
[Steroid use] has not been shown to impact efficacy as far as we can tell, so it’s definitely OK to use steroids.3-5,7,8,12,13
With everything else (PFS, OS, and so on) being similar, we have looked at quality of life. The questionnaires used were similar. Interestingly, both the KEYNOTE-426 trial of axitinib/pembrolizumab14 and the CLEAR trial of lenvatinib/ pembrolizumab15 showed similar quality-of-life [results for each of the experimental regimens vs] sunitinib.
The trial of cabozantinib/nivolumab stands out, because on both the questionnaires used, the FKSI-19 [Functional Assessment of Cancer Therapy-Kidney Symptom Index-19 Item Version] and the EuroQol-5 Dimension-3 Level, cabozantinib/nivolumab showed improvement over sunitinib in health-related quality of life.16
At this recent ASCO [American Society of Clinical Oncology] Annual Meeting, there was a presentation by Robert J. Motzer, MD, looking at whether quality of life had an impact on survival in metastatic RCC. He analyzed data from the CheckMate 214 [NCT02231749] trial of ipilimumab/nivolumab and showed that a 5-point improvement in quality of life can actually make a survival difference.17 That tells you that it is important for us to pay attention to patients’ quality of life in addition to looking at the efficacy numbers.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 3.2023. Accessed October 22, 2022. https://bit.ly/2TAx1m3
2. FDA approves nivolumab plus cabozantinib for advanced renal cell carcinoma. FDA. January 22, 2021. Accessed October 23, 2022. https://bit.ly/3igwGXM
3. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
4. Powles T, Choueiri TK, Burotto M, et al. Final overall survival analysis and organ-specific target lesion assessments with two-year follow-up in CheckMate9 ER: nivolumab plus cabozantinib versus sunitinib for patients with advanced renal cell carcinoma. J Clin Oncol. 2022;40(suppl 6):350. doi:10.1200/ JCO.2022.40.6_suppl.350
5. Apolo AB, Powles T, Burotto M, et al. Nivolumab plus cabozantinib (N+C) versus sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. J Clin Oncol. 2021;39(suppl 15):4553. doi:10.1200/JCO/2021.39.15_suppl.4553
6. Powles T, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for locally advanced or metastatic renal cell carcinoma (mRCC): phase III KEYNOTE-426 study. J Clin Oncol. 2019;37(suppl 7):543. doi:10.1200/JCO.2019.37.7_suppl.543
7. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): results from 42-month follow-up of KEYNOTE-426. J Clin Oncol. 2021;39(suppl 15):4500. doi:10.1200/JCO.2021.39.15_suppl.4500
8. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563-1573. doi:10.1016/S1470-2045(20)30436-8
9. Choueiri TK, Powles TB, Albiges L, et al. Phase III study of cabozantinib (C) in combination with nivolumab (N) and ipilimumab (I) in previously untreated advanced renal cell carcinoma (aRCC) of IMDC intermediate or poor risk (COSMIC-313). Ann Oncol. 2022;33(suppl 7):S808-S869. doi:10.1016/annonc/annonc1089
10. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
11. Choueiri TK, Powles T, Porta C, et al. A phase 3 trial of lenvatinib plus pembrolizumab versus sunitinib as a first-line treatment for patients with advanced renal cell carcinoma: overall survival follow-up analysis (the CLEAR Study). Oral presentation at: Kidney Cancer Research Summit 2021; October 7-8, 2021; Philadelphia, PA and Virtual. Accessed October 23, 2022. https://bit.ly/3b6uVta
12. Grünwald V, Powles T, Kopyltsov E, et al. Analysis of the CLEAR study in patients (pts) with advanced renal cell carcinoma (RCC): depth of response and efficacy for selected subgroups in the lenvatinib (LEN) + pembrolizumab (PEMBRO) and sunitinib (SUN) treatment arms. J Clin Oncol. 2021;39(suppl 15):4560. doi:10.1200/ JCO.2021.39.15_suppl.4560
13. Motzer RJ, Choueiri TK, Powles T, et al. Nivolumab + cabozantinib (NIVO+CABO) versus sunitinib (SUN) for advanced renal cell carcinoma (aRCC): outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. J Clin Oncol. 2021;39(suppl 6):308. doi:10.1200/JCO.2021.39.6_suppl.308
14. Bedke J, Rini B, Plimack E, et al. Health-related quality-of-life (HRQoL) analysis from KEYNOTE-426: pembrolizumab (pembro) plus axitinib (axi) vs sunitinib for advanced renal cell carcinoma (RCC). Presented at: 35th Annual European Association of Urology Congress; July 17-19, 2020; Virtual. Accessed October 23, 2022. https://bit.ly/3AXwEuq
15. Motzer R, Porta C, Alekseev B, et al. Health-related quality-of-life outcomes in patients with advanced renal cell carcinoma treated with lenvatinib plus pembrolizumab or everolimus versus sunitinib (CLEAR): a randomised, phase 3 study. Lancet Oncol. 2022;23(6):768-780. doi:10.1016/S1470-2045(22)00212-1
16. Cella D, Motzer RJ, Suarez C, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(2):292-303. doi:10.1016/S1470-2045(21)00693-8
17. Cella D, Hamilton M, Blum SI, et al. The relationship between health-related quality of life (HRQoL) and clinical outcomes in patients with advanced renal cell carcinoma (aRCC) in CheckMate (CM) 214. J Clin Oncol. 2022;40(suppl 16):4502. doi:10.1200/JCO.2022.40.16_suppl.4502

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen