Under Fasting and Fed Conditions, CC-90010 Efficacy Remains Consistent in Advanced Malignancies
Longer follow-up with parts A, B, and C of the phase 1 study CC-90010- ST-001 revealed continued safety and efficacy with the small molecule BET inhibitor CC-90010.
Victor Moreno, MD, PhD

Longer follow-up with parts A, B, and C of the phase 1 study CC-90010- ST-001 (NCT03220347) revealed continued safety and efficacy with the small molecule BET inhibitor CC-90010 in a presentation made virtually by Victor Moreno, MD, PhD, during the European Society for Medical Oncology Targeted Anticancer Therapies Virtual Congress 2021.
“Across parts A, B, and C, TRAEs were mostly mild and manageable with transient dose interruption and reductions,” Moreno said, lead author of the study and director of clinical research at START Madrid-Fundación Jimenez Diaz in Madrid, Spain.
“[Further], comparable exposures under fasting and fed conditions support dosing CC-90010 without consideration for food intake,” he continued.
The trial is comprised of a dose-escalation portion (part A; n = 69), a safety and efficacy portion (part B; n = 21), and a food-effect assessment (part C; n = 41). Patients in part A had advanced unresectable solid tumors (n = 67) or relapsed/ refractory diffuse large B-cell lymphoma (R/R DLBCL; n = 2). In part B patients had DLBCL, and patients in part C had advanced solid tumors.
Patients in part C were a median age of 58 years, and 11 patients were older than 65 years. Twenty-six (63%) patients had an ECOG performance status of 0, and 15 (37%) had a performance status of 1. The median number of prior therapies in this segment was 2, and at enrollment, patients were heavily pretreated
The pharmacokinetics for patients in part C demonstrated that fasting parameter estimates from part C were consistent with part A exposures.
“This portion of the analysis showed that Cmax [the maximum serum concentration that a drug achieves] was 20% lower in patients who were fed versus patients who were fasting,” Moreno said. Further, systemic exposures met bioequivalence criteria and did not appear to be affected by meal consumption. Moreno noted that both intra- and interpatient variability was the same under both fasting and fed conditions.
After pharmacodynamic analysis of CC-90010, the investigators measured messenger RNA (mRNA) levels of CCR1, a gene with expression not related to inhibition; Moreno said the modulation was dose and scale dependent.
CC-90010 doses over 25 mg caused more than a 50% decrease in CCR1 mRNA 4 hours after the last dose. CCR1 modulation was similar in parts A and B after the first and last dose at the recommended phase 2 dose.
Regarding treatment-related adverse effects (TRAEs) across all cohorts, most were mild or moderate, reversible, and easily manageable by dose adjustments and supportive care, Moreno said.
“The most common grade 3 or 4 TRAEs were thrombocytopenia, diarrhea, and nausea,” he said. “The high percentage of thrombocytopenia in part B reflects the high proportion of patients with hematologic malignancies and advanced disease.”
In part A, 6 (11%) patients experienced dose-limiting toxicities across dosing schedules. Overall, no treatment-related deaths occurred.
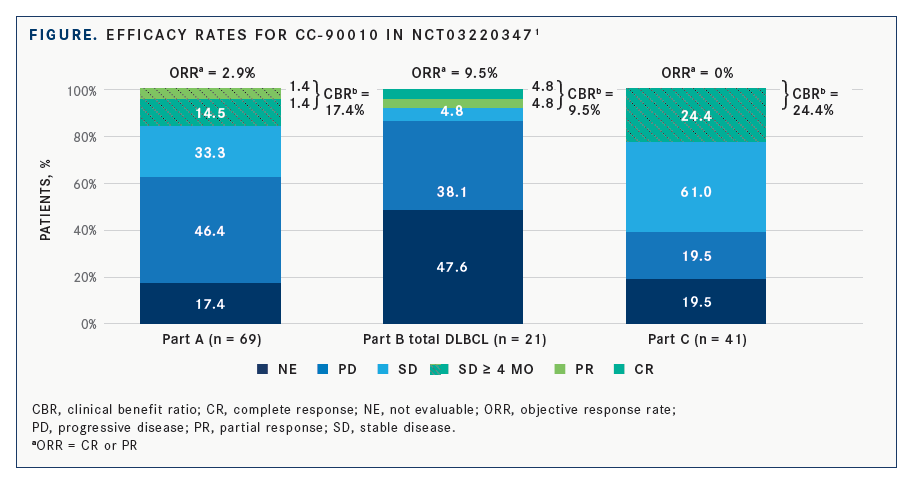
In terms of efficacy across the cohorts, in part A, 10 (14.5%) patients had stable disease for more than 4 months. One patient with progressing grade 2 astrocytoma had a complete response that lasted 19 cycles before progression, and 1 patient with endometrial carcinoma had a partial response for 8 cycles.
In part B, 3 patients with R/R DLBCL responded to treatment, with 1 complete response after completing 5 cycles, and 2 partial responses with 1 patient completing 3 cycles. Finally, in part C, 2 patients with refractory glioma had radiographic radio-graphic minor responses, including 1 patient who had been on treatment for 14 cycles (FIGURE).1
Moreno highlighted 2 cases of patients with brain malignancies. One patient in part A had a grade 2 diffuse astrocytoma and experienced a durable complete response lasting 19 cycles, as determined by metabolic and radiographic scans. He also highlighted a patient in part C with refractory glioma who experienced a radiographic minor response with 38% tumor shrinkage after 14 cycles. “There are further studies being conducted of this compound, specifically for brain tumors,” Moreno said.
As with parts A and B, CC-90010 in part C was well tolerated with promising preliminary antitumor activity in heavily pretreated patients with advanced malignancies, with promising responses in brain malignancies.
Reference:
Moreno V, Villar MV, Sanchez JMS, et al. CC-90010, a reversible, potent oral bromodomain and extraterminal inhibitor (BETi) in patients (pts) with advanced solid tumours (aSTs) and relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL): longer follow-up from parts A & B and first reporting of part C of a phase 1 study. Ann Oncol. 2021;32(suppl 1):S5. doi:10.1016/j.annonc.2021.01.021

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More