Targeting EZH2 Delivers Promising Activity in Lymphomas
Targeting EZH2, the catalytic subunit of the multiprotein PRC2, may represent an attractive therapeutic objective in malignant lymphoma, Vincent Ribrag, MD, told his audience at the American Association for Cancer Research’s inaugural Advances in Malignant Lymphoma meeting.
Targeting EZH2, the catalytic subunit of the multiprotein PRC2, may represent an attractive therapeutic objective in malignant lymphoma, Vincent Ribrag, MD, told his audience at the American Association for Cancer Research’s inaugural Advances in Malignant Lymphoma meeting.
EZH2 regulates B-cell maturation and cell fate and is a “major player and gatekeeper in the germinal center cell,” he said.EZH2loss-of-function mutations have been observed frequently in myelodysplastic syndrome and myeloproliferative neoplasms. Gain-of-function mutations, on the other hand, result in elevated H3K27me3 levels, and aberrant trimethylation of H3K27me3 is oncogenic in many cancers, including lymphoma.
Ribrag, a senior staff member and the chair of the hematology committee at Institut Gustave-Roussy in Villejuif, France, said there are 2 main strategies for EZH2 inhibition: (1) occupy and block the catalytic EZH2 site and (2) inhibit protein-protein interaction between the ectoderm development protein (EED) and EZH2, leading to loss ofEZH2function.
Investigators are just beginning to explore the potential of EZH2 inhibition as a treatment for lymphoma, but the early returns are promising.
“We can say that there is activity and the safety profile is rather good. The precise mechanism is not well understood, especially for patients with wild-typeEZH2,” Ribrag said. “What is important for us as clinicians is the duration of therapy. We don’t know for how long we have to give the drugs, but the percentage of complete response [has been] very low until now, which may mean that patients have to maintain the therapy for very prolonged times, which is something we have to check very carefully.”
Ribrag was the lead investigator for a first-in-human phase I dose-escalation trial evaluating the potent and highly selective EZH2 inhibitor tazemetostat in 58 patients with relapsed/refractory (R/R) cancers.1Thirty-seven patients with R/R solid tumors and 21 with R/R B-cell non-Hodgkin lymphoma (NHL) were assigned to doses from 100 to 1600 mg of daily tazemetostat. Of the patients with NHL, 14 had diffuse large B-cell lymphoma (DLBCL), 6 had follicular lymphoma (FL), and 1 had marginal zone lymphoma.
The median patient age was 63 (range, 24-84). Eight patients (38%) had received 3 prior treatments, 3 (14%) had received 4 prior treatments, and 7 (33%) had received ≥5 treatments. Three had received ≤2 prior treatments. Eight patients (38%) had undergone autologous hematopoietic stem cell transplant and 17 (57%) had undergone radiotherapy. There was no restriction according to mutation screening, Ribrag said.
At the November 2015 data cutoff, the overall response rate (ORR) was 62% for patients with NHL assigned to the 800-mg dose, compared with 50% at 1600 mg and 22% at <800 mg.
Observed reductions of the H3K27me3 signal were improved at higher doses but were identical at the 800-mg and 1600-mg dose levels.
Ribrag added that the drug was well tolerated. “Dose was increased, but we had no organ-specific toxicity to mention,” he said. “There were very few grade 3 or more toxicities even at the highest dose.”
Incidence of grade ≥3 treatment-emergent adverse events was 16% at the 800-mg doses, compared with 33% at 1600 mg and 29% at <800 mg. There was only 1 dose-limiting toxicity observed, a grade 4 thrombocytopenia at 1600 mg, and the maximum-tolerated dose was not reached. The recommended phase II dose was set at 800 mg per day.
Based on these results, investigators initiated an ongoing phase II trial for DLBCL and R/R FL in Western Europe, the United Kingdom, the United States, Canada, and Australia (NCT01897571). The prospective, 5-arm trial is evaluating frontline tazemetostat for elderly high-risk patients.
As of data cutoff, 45 enrolled patients hadEZH2-mutant FL and 45 hadEZH2wild-type FL. In the DLBCL group, there were 60 patients with germinal center B-cell (GCB)EZH2-mutated disease, 60 with GCBEZH2wild-type, and 60 with non-GCB disease.
Patients with FL had received a median of 4 prior treatments; those with DLBCL had received 3. Fifty-four percent of patients withEZH2-mutant FL were refractory to their last prior regiment, compared with 48% for wild-type, 82% forEZH2-mutant DLBCL, and 63% for wild-type DLBCL.
As of the June 1, 2017, data cutoff, the ORR for patients withEZH2-mutated FL was 92%, but just 26% in patients with wild-type FL.2The ORR was 29% for those withEZH2-mutant DLBCL and 15% for those with wild-type DLBCL (TABLE).
“It was active in [patients with] follicular lymphoma with the mutation, less active in the wild-type [population],” Ribrag said, adding that the combination appeared safe and well tolerated.
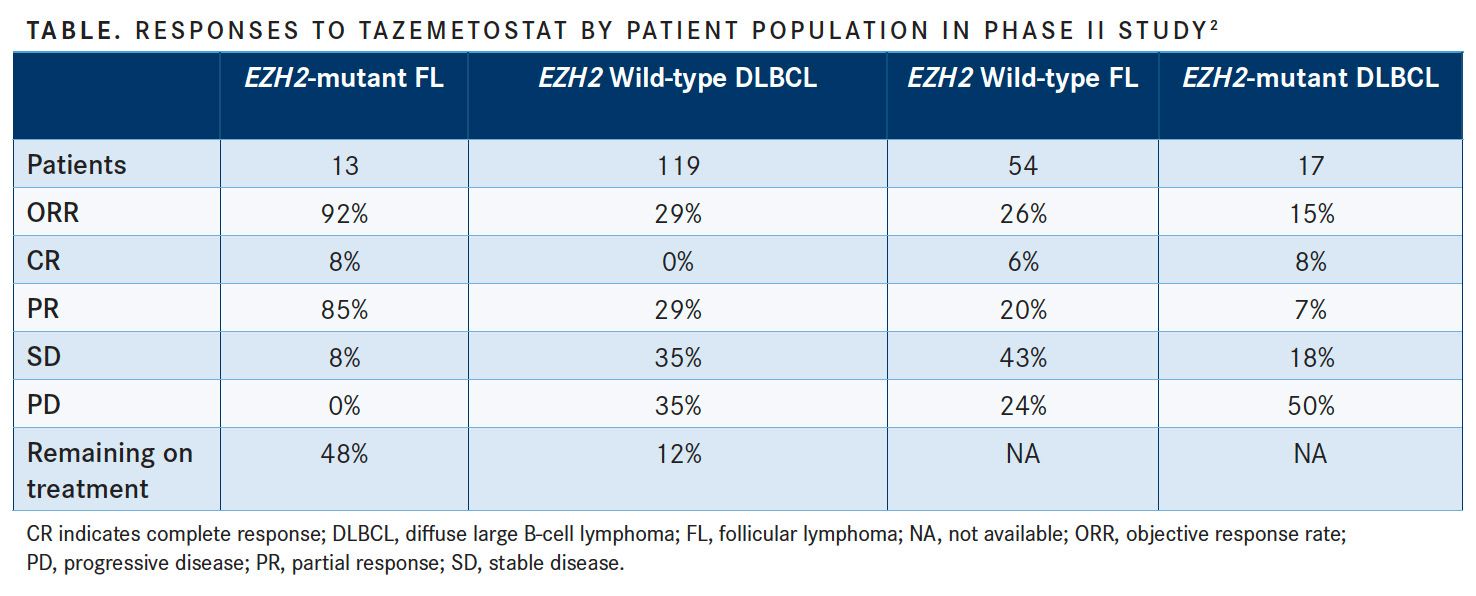
“What was also important in this study is that the toxicity profile was the same as what we observed in phase I,” he said. “Almost all patients remain on study; we have just 3% of patients who left the study because of safety issues.”
However, the FDA placed a halt on enrollment into clinical trials with tazemetostat in April 2018 after Epizyme, the manufacturer of the agent, noted that a secondary malignancy was developed in one of the patients on a pediatric phase I trial. Patients already on the trial were allowed to continue.
For the purpose of achieving a more active delivery, investigators performed next-generation analysis on archive tumor and circulating tumor DNA collected from a subset of 92 patients from the phase II trial.3They determined that EZH2- andMYD88-activating mutations appeared to be positive predictors and may be independent mechanisms of sensitivity to tazemetostat. Mutations inMYC,TP53, andHIST1H1Eappeared to be negative predictors.
Data in 82 patients with R/R FL available for safety analysis in the phase II study have been updated. As of the May 1, 2018, data cutoff,EZH2-activating mutations had been detected in 17% (n = 248) of patients.4Ribrag saidEZH2activating mutations are a good prognostic marker in FL and are associated with good outcomes with R-CHOP (rituximab [Rituxan] plus cyclophosphamide, doxorubicin, vincristine, and prednisone) treatment.
Accrual closed for the FL wild-type arm with 54 patients available for efficacy analysis, 28 withEZH2-mutated FL.
The ORR was 71% (95% CI, 51%-87%) amongEZH2-mutated patients with FL and 33% (95% CI, 21%-47%) among wild-type patients. Three mutated patients (11%) and 3 wild-type patients (6%) had complete responses.
The median time to response was 11.9 weeks in the mutated group and 15.9 weeks in the wild-type group. The median duration of response was far greater in the wild-type group (76.0+ vs 32.3+ weeks), but the percentage of patients with ongoing responses was similar (56% vs 55%, respectively).
Overall, 38 of 82 patients (46%) had an objective response and 66 of 77 (85.7%) experienced a reduction in tumor burden. Median progression-free survival among responders was 48.6+ weeks in the mutated arm and 84.3+ weeks in the wild-type arm.
“Most patients remain [in the] study,” Ribrag said. “We have to follow them a little bit more to see if we can reach a complete response.”
On the other hand, Epizyme announced on August 2 that the phase II trial would be halted for the cohort of patients with R/R DLBCL as the company found that there was not sufficient activity seen in these patients to validate continued research in this area.
Ongoing Phase I Studies
Ribrag mentioned 3 agents, the EZH2 inhibitors CPI-1205 and GSK2816126 and the EED inhibitor MAK683, under investigation in phase I studies. Preliminary results in 22 evaluable patients with B-cell lymphoma showed that CPI-1205 is associated with tumor shrinkage, whereas GSK2816126 has been well tolerated in patients with hematologic malignancies and has demonstrated promising antitumor activity.5,6
MAK683 is the first drug to target the interaction between EED and EZH2. The agent selectively binds to EED to block the EED-EZH2 interaction, which led to reduced tumor cell proliferation inEZH2-mutated and PRC2-dependent tumor cells in preclinical studies.
MAK683 is under investigation in the first-in-class and first-in-human phase I/II study CMAK683X2101 (NCT02900651). Investigators are planning to recruit 113 patients with a range of advanced cancers including DLBCL, nasopharyngeal carcinoma, and sarcoma.
References:
- Italiano A, Soria JC, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018;19(5):649-659. doi: 10.1016/S1470- 2045(18)30145-1.
- Morschhauser F, Salles GA, McKay P, et al. Interim report from a phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory B-cell non-Hodgkin lymphomas. Hematol Oncol. 2017;35(suppl S2):24-25. doi: 10.1002/hon.2437_3.
- Blakemore SJ, Daigle SR, McDonald AA, et al. Preliminary evidence of a molecular predictor of tazemetostat response, beyond EZH2 mutation, in NHL patients via characterization of archive tumor and circulating tumor DNA. Hematol Oncol. 2017;35(S2):156-160. doi: 10.1002/hon.2438_14.
- Morschhauser F, Tilly H, Chaidos A, et al. Interim update from a phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory (R/R) follicular lymphoma (FL). Presented at: 2018 EHA Congress; June 14-17, 2018; Stockholm, Sweden. Abstract S100.
- Harb W, Abramson J, Lunning M, et al. A phase 1 study of CPI-1205, a small molecule inhibitor of EZH2, preliminary safety in patients with B-cell lymphoma. Ann Oncol. 2018;29(suppl 3; abstr 420). doi: 10.1093/annonc/mdy048.001.
- Yap TA, Winter JN, Leonard JP, et al. A phase I study of GSK2816126, an enhancer of zeste homolog 2(EZH2) inhibitor, in patients (pts) with relapsed/refractory diffuse large B-cell lymphoma (NLBCL), other non-Hodgkin lymphomas (NHL), transformed follicular lymphoma (tFL), solid tumors and multiple myeloma (MM). Blood. 2016;128(22):4203.

Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen