TAPUR Results Roll Out, Bringing Potential New paradigms Into Focus
When patients with locally advanced or metastatic soft tissue sarcoma do not respond to standard chemotherapy, they face a difficult situation.
Scott Michael Schuetze, MD, PhD

When patients with locally advanced or metastatic soft tissue sarcoma (STS) do not respond to standard chemotherapy, they face a difficult situation.
“There are very few FDA-approved treatments for patients with soft tissue sarcoma,” Scott Michael Schuetze, MD, PhD, a clinical professor for the University of Michigan Health at Michigan Medicine, said.
Doxorubicin (Lipodox) is generally the first chemotherapy offered. Other drugs, such as trabectedin (Yondelis) and eribulin mesylate (Halaven), are also approved for certain patients, but such therapies come with significant risk of adverse effects. Although it’s a short list, it may soon grow.
“Over the past 10 to 20 years, researchers have identified molecular abnormalities in sarcomas that may be vulnerable to disruption by drugs targeting specific growth pathways, and pharmaceutical companies have done a remarkable job in developing pharmaceuticals to target these pathways,” Schuetze explained.
Of course, developing such drugs is one thing, getting them to patients who need them can be quite another.
TAPUR Design First launched in 2016, TAPUR (NCT02693535) is a phase 2, open-label, basket study sponsored by the American Society of Clinical Oncology (ASCO) that allows investigators to evaluate how small cohorts of patients with tumors having the same or similar genetic variations will respond to targeted therapies already approved for other indications. The study uses next- generation sequencing (NGS) to match patients with potentially actionable mutations to promising therapies and study cohorts. Cohorts begin with up to 10 patients who have the same cancer type, genomic alteration, and study drug. If 2 or more patients appear to respond to the therapy, the cohort can be expanded to 28 enrollees.1
Currently, TAPUR is enrolling patients at 141 sites, and 19 drugs are accessible through partnerships with drug developers (TABLE1).
One of those drugs is palbociclib (Ibrance), a therapy approved for the treatment of hormone receptor–positive, HER2-negative breast cancer. At 2021 ASCO Annual Meeting, Schuetze and colleagues reported on the use of palbociclib in patients with STS and CDK4 amplification.2 Each of the patients in the study had either not responded to or progressed following treatment.
“Most of the patients treated in this cohort had a life expectancy of less than a year, and identification of more effective and better-tolerated treatments for these patients is a priority,” Schuetze said.
Earlier work has identified CDK4 and MDM2 as overexpressed and pathologically important for the tumor type, Schuetze said. TAPUR gave investigators the ability to determine responses in a small group of patients with STS.
In their TAPUR study, 1 patient had a partial response and 12 patients had stable disease at 16-plus weeks (SD16+). That translated to a disease control rate (DCR) of 48% (95% CI, 31%-62%) and an objective response rate (ORR) of 3.7% (95% CI, 0.1%-19%). Schuetze and colleagues said 9 of the 13 patients with disease control continued on treatment for more than 32 weeks.
“Most of the patients who enrolled in the study had dedifferentiated liposarcoma, and the results in this group were in line with expectations and results from prior studies, although it was disappointing that we did not see objective tumor responses in this sarcoma subtype,” Schuetze said. “The objective response in a patient with soft tissue sarcoma that was not a dedifferentiated liposarcoma was a bit of a pleasant surprise.”
Further, adverse events (AEs) were consistent with previous studies, he said.
Following in NCI’s Footsteps
Ajjai Alva, MBBS, another TAPUR investigator from Michigan Medicine, noted that TAPUR is not the first time a trial has attempted to use NGS sequencing to identify patients for quick entry into trials. The National Cancer Institute (NCI) launched the NCI-MATCH trial (NCT02465060) in 2015 using a similar model of matching patients via genetic mutations. However, Alva said that study had some limitations that TAPUR does not. For instance, in NCIMATCH, the sequencing was done at a single center to ensure uniformity in the sequencing.
“They really placed an importance on the stringency of the sequencing, which is good for science,” he said. However, it also resulted in procedural bottlenecks. “So logistically, it was very difficult.” TAPUR offers more flexibility in terms of where sequencing is done and which platforms are used. That makes the process of enrolling patients and beginning therapy significantly faster, something that is key for patients who often face progressing disease.
Latest Results
Schuetze’s study was not the only news to come out of the ASCO meeting. As TAPUR reached its 5-year anniversary, a number of new results were presented, including reports on head and neck cancer and uterine cancer.
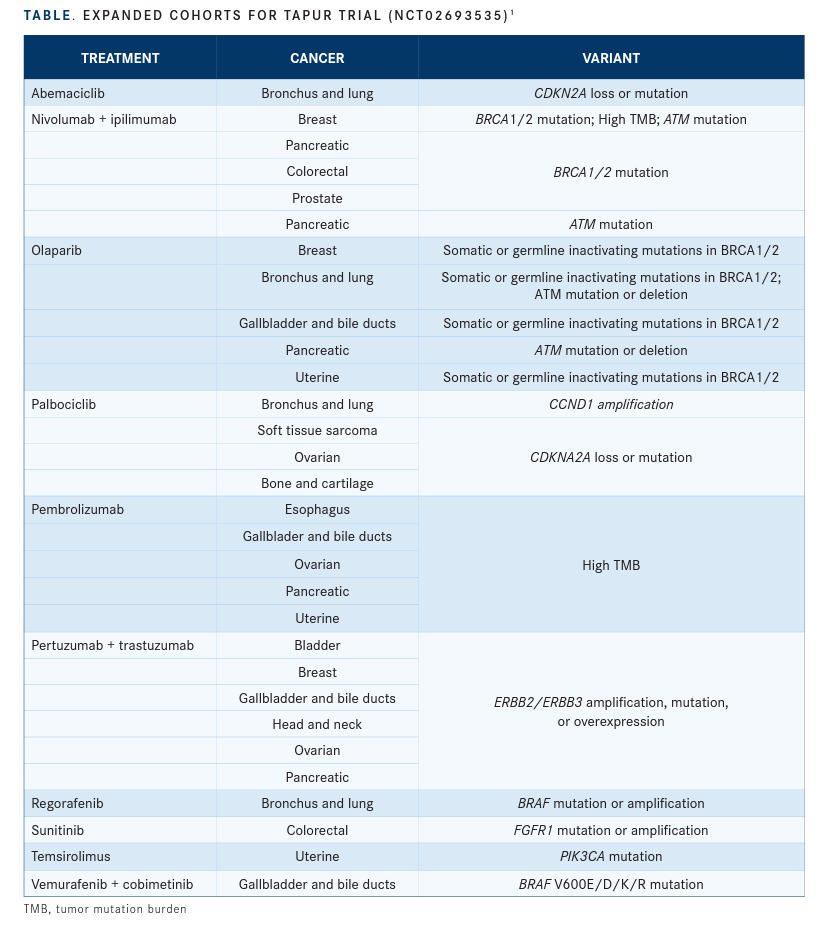
In another palbociclib study, Evan Pisick, MD, of Cancer Treatment Centers of America, and colleagues evaluated the drug in a cohort of patients with head and neck cancer and CDKN2A mutation (8 patients) or loss (20 patients).3 Like the patients in Schuetze’s study, these patients had no standard treatment options and advanced disease.
Nine of the 20 patients with CDKN2A loss achieved SD16+, and 1 patient with a CDKN2A mutation achieved the same. The DCR was 37% (95% CI, 21%-50%).
Half the patients (14) experienced at least one grade 3 to 5 AE or serious AE, the most common of which was low white blood cells/platelets. One patient experienced grade 5 respiratory failure, which investigators said was most likely due to extensive lung metastases and aspiration, although they noted that palbociclib-related pneumonitis could not be ruled out.
Additionally, Hussein Moustapha Ali- Ahmad, MD, of the Michigan Cancer Research Consortium, reported on a cohort of patients with uterine cancer who had ERBB2 or ERBB3 amplification, overexpression, or mutations.4 The 28 patients all had measurable disease and no standard treatment options. Most of the patients (21) had ERBB2 amplification; 4 patients had ERBB2 mutations; 1 had ERBB2 overexpression; 1 had ERBB2 amplification and mutation; and 1 had ERBB3 amplification. They were treated with pertuzumab (Perjeta) and trastuzumab (Herceptin).
Two participants with ERBB2 amplification had partial responses, and 7 achieved SD16+. One patient with ERBB2 8421 mutation but without amplification also achieved SD16+. That translated to a DCR of 37% (95% CI, 21%50%) and an ORR of 7.1% (95% CI, 0.8%-24%). Just 1 patient experienced a grade 3 AE (muscle weakness), which investigators said was possibly related to the therapy.
Building on Earlier Work
The combination of pertuzumab and trastuzumab was also the subject of research presented last year at the ASCO Gastrointestinal Cancers Symposium. That report evaluated the combination therapy’s ability to treat patients with colorectal cancer and ERBB2 amplification.5 One patient in the study also had a mutation of the gene. The results of the trial produced better DCR (50%) and ORR (25%) versus Ali-Ahmad’s and colleagues’ new uterine cancer study.
Other 2020 results included promising outcomes for patients with prostate cancer and BRCA1/2 inactivating mutations who were treated with olaparib (Lynparza).6 That study found 68% of patients achieved disease control and 36% of patients had an objective response.
However, olaparib did not perform as well in patients with pancreatic cancer and BRCA1/2 inactivating mutations. In that study, 31% and 4% of patients achieved disease control and objective response, respectively.7
Alva’s research involved looking at pembrolizumab (Keytruda) in patients with metastatic breast cancer who had high tumor mutational burden (TMB). The study found TMB was a meaningful biomarker predictive of response to pembrolizumab, leading to a DCR of 37% and ORR of 21%.8 Median progression-free survival was 10.6 weeks, and median overall survival was 30.6 weeks.
Alva said that before TMB was identified, the protein PD-L1 was used as a biomarker, but it was imprecise.
“A lot of patients in every cancer type do not express or do not have detectable PD-L1 and still respond,” he said. “And the other way around also is true: Sometimes there is high PD-L1 expression, and those tumors also do not respond.”
The TAPUR study showed TMB, although not a perfect biomarker, could help identify a cohort of patients in whom a significant proportion would respond to the therapy.
A similar study of pembrolizumab found DCR and ORR of 28% and 4%, respectively, in patients with metastatic colorectal cancer and high TMB.9
Generating Impact
Shortly after those studies were released, the FDA approved pembrolizumab for patients across cancer types if they have high TMB, progressed despite treatment, and have no other available treatment options.10
The expanded approval was based on the KEYNOTE-158 trial.10 Alva noted that the TAPUR studies are not designed to lead to FDA action—with just 28 patients they generally are too small—but they can quickly generate data sufficient to trigger larger studies.
“The study was never designed to back FDA regulatory approval,” he said. “It was merely to find signals, quick signal checking—does TMB hold promise?”
Schuetze added that TAPUR allows for the investigation of therapies for cancer types that might otherwise be too rare to be the subject of a major study.
“These cancers studied in TAPUR are, for the large part, too rare for study in larger randomized phase 2 or phase 3 trials, and TAPUR is an innovative mechanism to collect treatment data from patients with very rare cancer subtypes,” he noted.
Schuetze said because the trials are not randomized makes it difficult to determine the impact of what the TAPUR study will be, but he added that it is already serving a meaningful opportunity “to gain knowledge about new treatment options and further medical care for patients with rare cancers.”
As for what it all means long term, Alva said he is a big believer in NGS as a tool that will play a major role in the future of cancer care.
“Biology wise, it gives us a lot of clues about prognostic markers, for example, that some things are going to work, some things are more likely to work, and some things are never going to work,” Alva said. “Those kinds of information—the more information, the better for us.”
REFERENCES
1. News & updates. ASCO TAPUR. Updated April 26, 2021. Accessed July 1, 2021. https://bit.ly/2VfDolw
2. Schuetze S, Rothe M, Mangat PK, et al. Palbociclib (P) in patients (pts) with soft tissue sarcoma (STS) with CDK4 amplification: results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study. J Clin Oncol. 2021;39(suppl 15):11565. doi:10.1200/JCO.2021.39.15_suppl.11565
3. Pisick EP, Rothe M, Mangat PK, et al. Palbociclib (P) in patients (pts) with head and neck cancer (HNC) with CDKN2A loss or mutation: results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study. J Clin Oncol. 2021;39(suppl 15):6043. doi:10.1200/jco.2021.39.15_suppl.6043
4. Ali-Ahmad HM, Rothe M, Mangat PK, et al. Pertuzumab plus trastuzumab (P+T) in patients (Pts) with uterine cancer (UC) with ERBB2 or ERBB3 amplification, overexpression or mutation: results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study. J Clin Oncol. 2021;39(sup-pl 15):5508. doi:10.1200/jco.2021.39.15_suppl.5508
5. Gupta R, Garrett-Mayer E, Halabi S, et al. Pertuzumab plus trastuzum-ab (P+T) in patients (Pts) with colorectal cancer (CRC) with ERBB2 am-plification or overexpression: results from the TAPUR study. J Clin Oncol. 2020;38(suppl 4):132. doi:10.1200/jco.2020.38.4_suppl.132
6. Pisick EP, Garrett-Mayer E, Halabi S, et al. Olaparib (O) in patients (pts) with prostate cancer with BRCA1/2 inactivating mutations: results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study. J Clin Oncol. 2020;38(suppl 15):5567. doi:10.1200/jco.2020.38.15_sup-pl.5567
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More