Olaparib Improves Disease–Free Survival in Early HER2-Negative Breast Cancer
Patients who recived olaparib after a median follow-up of 2.5 years revealed a 42% reduction in invasive disease–free survival, including local and metastatic recurrence of breast cancer, other new cancers, and death due to any cause.
Andrew Tutt, PhD, MB ChB

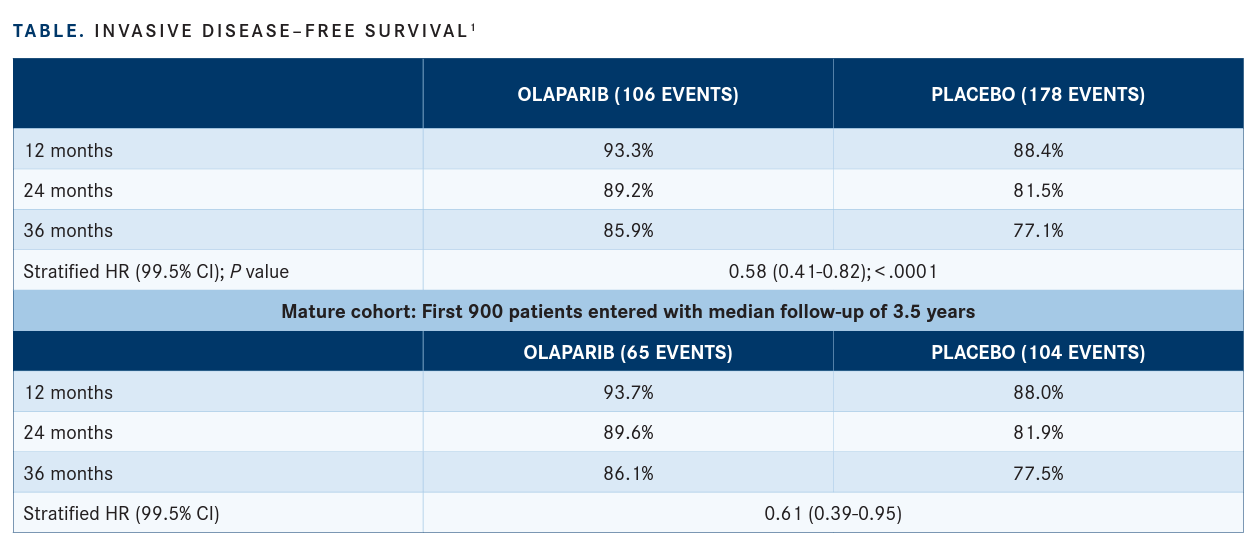
Patients who recived olaparib (Lynparza) after a median follow-up of 2.5 years revealed a 42% reduction in invasive disease–free survival (iDFS), including local and metastatic recurrence of breast cancer, other new cancers, and death due to any cause, (hazard ratio [HR], 0.58; 99.5% CI, 0.41-0.82; P < .0001), according to findings from the phase 3 OlympiA trial (NCT02032823; TABLE, page 37).1 Additionally, investigators noted a difference in 3-year iDFS rate between olaparib and placebo of 8.8% (95% CI, 4.5%-13.0%; stratified HR, 0.58; 99.5% CI, 0.41-0.82; P < .0001).
During a virtual press briefing ahead of the 2021 American Society of Clinical Oncology Annual Meeting, investigators reported clinically meaningful benefit 1 year after initiating standard of care therapies, such as surgery, chemotherapy, hormone therapy, or radiation therapy, in patients with BRCA1/2-mutant, early HER2-negative breast cancer who are at high risk for recurrence.
“The OlympiA study is the first to report the ben-efits of [an adjuvant] PARP inhibitor for early forms of germline BRCA1/2-mutation–associated cancer,” said presenting study author Andrew Tutt, PhD, MB ChB, head of the Division of Breast Cancer Research and director of the Breast Cancer Now Toby Robins Research Centre at the Institute of Cancer Research and Guy’s Hospital King’s College, London, United Kingdom, a clinician scientist with the Laboratory and Clinical Trials program, and a consultant clinical oncologist. “Patients who received olaparib after surgery and chemotherapy were more likely to be alive without cancer, [as well as] avoid metastasis, than the patients who received placebo.”
Patients with HER2-negative early breast cancer who harbor a BRCA1/2 mutation, present in approximately 5% of all breast cancers, are at high risk of disease recurrence. Although outcomes have been positive for patients with these mutations who have received standard treatments such as surgery, radiotherapy, and chemotherapy, the risk of recurrence remains high for some patients. As such, additional novel targeted therapies are needed to reduce the risk of recurrence in this patient population.
Olaparib is a PARP inhibitor that targets DNA repair defects in certain germline-mutant can-cers and was previously approved by the FDA in January 2018 for the treatment of patients with germline BRCA-positive, HER2-negative metastatic breast cancer. As such, investigators sought to examine the agent in patients with germline BRCA–mutated, HER2-negative early breast cancer.
The trial enrolled 1836 patients with HER2-negative breast cancer harboring a germ-line BRCA mutation who were randomized 1:1 to receive either 300 mg of oral olaparib (n = 921) twice daily for 1 year or placebo (n = 915). Additionally, patients needed to have been treated for early stage (stage II-III) breast cancer and have completed surgery and chemotherapy, with or without radiotherapy. Inclusion criteria also required that patients have a high risk of disease recurrence. Those who had received prior treatment with a PARP inhibitor were not eligible to enroll.
The primary end point for the study was iDFS; secondary end points included distant disease–free survival (DDFS), overall survival (OS), health-related quality of life, and safety. “Stringent criteria were applied for a planned interim analysis,” Tutt said. “At this analysis, OlympiA’s Independent Data Monitoring Committee found [that] these stringent criteria were met for early reporting.”
Additional findings indicated that after a median follow-up of 2.5 years, patients who were treated with olaparib experienced a 43% reduction in DDFS, including metastatic disease, new cancer, and death due to any cause (HR, 0.57; 99.5% CI, 0.39-0.83; P < .0001). The difference in 3-year DDFS rate between olaparib and placebo was 7.1% (95% CI, 3.0%-11.1%; stratified HR, 0.57; 99.5%, 0.39-0.83; P < .0001).
At the time of the early primary end point report, OS data were considered immature. However, although fewer deaths were reported in patients receiving olaparib vs placebo, OS was not significantly different between the 2 study cohorts at a median follow-up of 2.5 years (HR, 0.68; 99% CI, 0.44-1.05; P = .024). Moreover, the difference in 3-year OS rate between the 2 arms was 3.7% (95% CI, 0.3%-7.1%).
In terms of the safety profile, the adverse effects (AEs) reported in the olaparib group were consistent with what has been previously reported with the agent. Additionally, olaparib did not increase serious AEs, including hospital admissions or occurrences of other cancers such as leukemia. However, grade 3 or higher AEs were reported more often in patients receiving treatment with olaparib, and included anemia (9%), neutropenia (5%), leukopenia (3%), and fatigue (2%).
The most common AEs of any grade reported in patients who received olaparib included nausea (57%), fatigue (40%), anemia (23%), vomiting (23%), and headache (20%). Moreover, the most common any-grade AEs in the placebo arm were fatigue (27%), nausea (23%), headache (17%), diarrhea (14%), and arthralgia (12%).
REFERENCE
Tutt A, Garber J, Kaufman B, et al. OlympiA: a phase III, multicenter, ran-domized, placebo-controlled trial of adjuvant olaparib after (neo)adjuvant chemotherapy in patients with germline BRCA1/2 mutations and high-risk HER2-negative early breast cancer. Presented at: 2021 ASCO Annual Meeting; June 4-8, 2021; virtual. Abstract LBA1. Accessed July 1, 2021. https://bit.ly/2TOpnux
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More