Roundtable Discussion: Weiss Discusses Treating RET-Fusion Mutations in NSCLC
Jared Weiss, MD, led a discussion about a 59-year-old patient with RET-fusion-positive non–small cell lung cancer during a Targeted Oncology Case-Based Roundtable event.
Jared Weiss, MD

Jared Weiss, MD, associate professor, School of Medicine, section chief, Thoracic and Head and Neck Oncology, UNC-Chapel Hill/Lineberger Comprehensive Cancer Center, led a discussion about a 59.year-old patient with RET-fusion-positive non–small cell lung cancer (NSCLC) during a Targeted OncologyTM Case-Based Roundtable event.
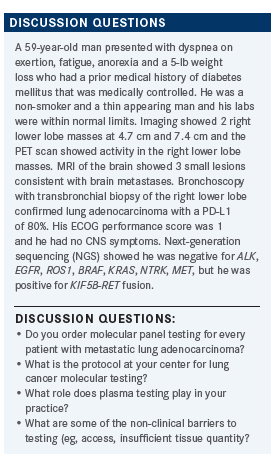
FEAGIN: Currently, we have a lung panel that that our pathology group automatically kicked in, and its PD-L1, ALK, and EGFR. After that, you have to ask for things, or you can do NGS.
WEISS: If you don’t get back anything, what are you tending to get? Is it subsequent al a carte FISH tests, or is it NGS? And if so, who’s panel?
FEAGIN: I tend to do NGS, and I use Tempus, myself.
WEISS: Anyone have anything to add to that, or differences in practice?
GADDE: We use a targeted NGS that goes out to [the Mayo Clinic], so it includes ALK, ROS, EGFR, MET, RET, and so on. It’s pretty comprehensive.
RUDOLPH: I just use a wide panel with Caris. Really, same across all tumor types, I don’t send a specific panel for each tumor type.
WEISS: Anyone getting plasma testing?
MANDALAPU: We do plasma testing in patients who have insufficient tissues, and the patient cannot have a re-biopsy for some reason. One other barrier that I have seen most of the times with NGS is, when doing the bronchoscopy, we’re seeing most of the time insufficient tissue as opposed to CT-guided biopsies. I don’t know if that is just my practice, or in general. That’s what I’ve observed in the last couple of years right now. Most of the times, if it is accessible, we are encouraging a CT-guided biopsy if the patient can have it, unless they have some emphysematous changes or something like that.
WEISS: It’s an interesting conversation, because I’ve had that conversation with colleagues, both in academia and in practice, across the country. It’s amazing how much geographic disparity there is in what’s easy to get, and in the skill level of getting it. In the case of endobronchial ultrasound [EBUS], I think the skill of the operator is highly, highly variable. Some people are reporting they never get sufficient tissue when their pulmonologist does EBUS, and others that’s almost never an issue. I think, also, the skill of the pathologists helps. If you have a cytologist on site, doing ROSE and telling you if there’s good tumor yield or not, that’s also quite helpful. Whereas, if your proceduralist is just having to guess, maybe a little less so.
RESKE: I use for sure more liquid biopsy, just to the constraints of getting biopsies done. Obviously, there’s some patients who are referred to us, and who are just very reluctant, who have insufficient tissue and then say, “There’s no way I will have another biopsy.”
WEISS: We’ve spoken some about some of the clinical barriers to testing. Are there non-clinical barriers like, for example, access, or resistance to certain types of biopsies?
RUDOLPH: I’m always a little bit unsure of whether sending both tissue and plasma will get covered, simultaneously. I’ve done it at progression, where I’ve done tissue at the initial diagnosis and plasma at subsequent on progression, and that does get covered for sure. Would love your thoughts if they’ve sent both simultaneously and experienced any coverage issues.
WEISS: In these conversations, what I’ve heard cited the most as a driver for getting liquid are the barriers with tissue: insufficient specimen, or the patient doesn’t want to get it. In my practice, my problem that drives me to use it sometimes is speed. There are issues with what the pathologist would call preanalytic variables, and I would call nonsense, but it can take 2-plus weeks to get tissue into a FedEx envelope. Whereas, if I draw blood for a liquid test, that goes off quickly, and most of those labs are rather quick.
One of the commercial vendors is as quick as a few days. In my practice, I tend to use liquid because I can pick the low-hanging fruit like EGFR quickly, for patients that are high probability. My tumor board was asking who wants to biopsy his patient is like throwing a steak into the lion’s den at my tumor’s board. The surgeon, and 3 interventional pulmonologists, and the VIR guy are clambering over each other for who gets to do it. On the comment of liquid and solid, there are some of the vendors that have both are offering a guarantee that the patient will only get 1 bill. I find that a practical thing for some situations, where you can sort of have the best of both worlds…You can’t action any oncogene unless it is discovered, and it can’t be discovered unless it’s tested.
MANDALAPU: I have 1 other concern. We know all these patients are stage IV, some of them has poor performance status, the rapidly progressing diseases. It’s difficult after 2 weeks of waiting, and then they say, “You don’t have enough tissue.” At that time, you are compelled to start on something, [such as] sending the tissue again. That’s what we have seen in our barrier in the communication. Once the tissue is sent, we don’t get any communication from them, unless we chase after them, “Hey, what happened, do we have the result yet”? So that, I think the process itself is sometimes time consuming.
MERRICK: I’ve certainly found that the practice patterns have been evolving and changing quite rapidly. I’d be interested in other’s opinions. Have we reached the point where NGS testing is now considered standard of care in metastatic lung adenocarcinoma? And, if that’s the case, should we also be getting both tissue and liquid testing since they often can complement each other?
GADDE: For not just metastatic disease, but I think even certain patients with stage II and III even. Certainly, I can’t comment that it’s being covered well in every case, I don’t know that, but we’re trying to send it. So yes, I would say NGS is standard.
MATRANA: In the state of Louisiana, we’re working on legislation as we speak that requires every insurer to cover complete NGS for any patient diagnosed with any cancer. It just passed our state senate and going to our [House of representatives]. You’d be surprised how open the legislators were to this. Even our conservative insurance-friendly legislators were very, very open to this when we brought it to them and said, “This is what we think cancer patients need.” They said, “We need cancer doctors telling us what cancer patients need. We’re tired of insurance companies telling us what cancer patients need.” I’d encourage anybody in other states to work towards legislating this sort of thing.
WEISS: I’ll accept anyone who wants to virtually throw tomatoes at this being an excessively academic perspective, but I’m going to go ahead and say that not only is NGS the optimal standard of care where available. I think when we start to talk about RET, where not all the fusion partners are always known, or NTRK, which is a larger rearrangement, that it’s not only next-gen, but next-gen with a good RNA fusion panel. I realize how painful this is to have to dig this far into the weeds, but these are weeds that I’ve seen matter in my practice. I’ll share that my practice yields a certain perspective, because [for few of my patients I’m] their first doctor. It’s bread and butter in my practice that there’s been a la carte testing, and it varies how much is done. EGFR, ALK, ROS is a common combination. I’ll send off next-gen with an RNA fusion panel, and 2 things are notable about the outcome.
One is how shockingly often that yields fruit. It’s like a knee-jerk reaction in a tertiary care practice to do this, but it gets places. We’ll often find RET and NTRK that were not previously found. That’s not surprising, that’s what I send it for. I send it for the NTRK, the RET, the out-there stuff.
What’s really surprising to me is that you occasionally find a EGFR and ALK that were previously tested, and were negative. And, my molecular pathology colleagues tell me that there actually is a little more sensitivity on next-gen, even for the classical changes, that that’s not shocking to them. So, I think it actually matters a lot. You actually find things. It’s not just an exercise.
I would comment that I think in these kinds of conversations, or at least in lectures, many of us who lecture a lot are hesitant to use brand names for testing, and instead we’ll say things like “broad testing,” which is what the guidelines say. But, we’re here in a group discussion, not in a lecture, so my apologies to my targeted oncology colleagues if this gets me in trouble, but there are 3 major commercial platforms that get you DNA next-gen with a good RNA fusion panel.
There’s Tempus, there’s Caris, and FoundationOne if you order the “heme” panel that gets you the good RNA fusion analysis. Those are the three easy-to-get commercial ones that I’m aware of. I’m sorry if that breaks any rule for endorsing 1 business or another. I have no conflict of interest with any lab testing company, I just think this matters a lot.
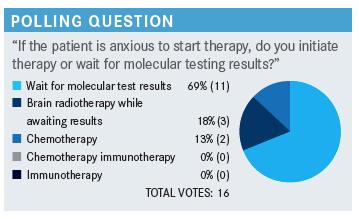
WEISS: I don’t know the right answer here. I think all the answers that were chosen are quite reasonable. We’ve never had a study in lung cancer to show that you harm survival by giving chemotherapy first, even though TKI is much more effective. There’s never been a sequence effect on survival, probably because crossover is so perfect. Using a little stereotactic while awaiting the results is perfectly reasonable. I don’t love to do it, in my opinion, which is a little biased against it, because these patients live more than long enough to get radio-necrosis in their brains, and so many of our TKIs have great efficacy in the brain.
I think it’s a good way to buy some patience, if you have to, with low risk to the patient, at least in the short and intermediate term. In personal, I’m the stickler, I’m going to wait for the results, especially in a case like this with high probability. The other answers that were chosen are all eminently reasonable. This is more of an opinion question than a right one. Anyone have any commentary, or questions?
MATRANA: I always tell patients who want to start therapy right away, that we can rush into things like a bull in a china shop and do it wrong, or we can be prudent and and I’ve never had anybody take me up on let’s rush in and do it wrong.
WEISS: It’s usually a failed attempt at comedy, as most of mine are, but I tell my patients that I’m a recovering New Yorker, and I’m as impatient as they are, but that while time matters a lot, it’s time to doing something that works that matters, not time to doing something. That line has been very effective in buying patience from patients.
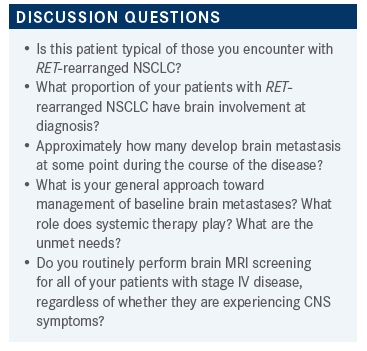
MANDALAPU: I have had [patients that were a prior smoker] he was still smoking when he was diagnosed with [RET-rearranged NSCLC]. I don’t know if this is something unusual, however.
WEISS: Well, we’ll look at the demographics of the trial data. I found all kinds of EGFR, ALK, RET, ROS1 in patients who’ve heavily smoked. There’s nothing about smoking that protects you against a nonsmoker’s cancer. So, what about the brain metastases? Are we comfortable with systemic therapy alone here?
FEAGIN: Right now, the patient appears to be asymptomatic, and just sort of dependent on the bulk of disease. Sometimes, if there is a bulkier disease, or if the patient’s more symptomatic as far as breathing, we might defer radiotherapy to the brain, and just treat viscerally or systemically first.
GADDE: Did your trial subgroup [of patients with brain metastases that] haven’t just looked at all the subgroups fully?
WEISS: We have some data coming up.
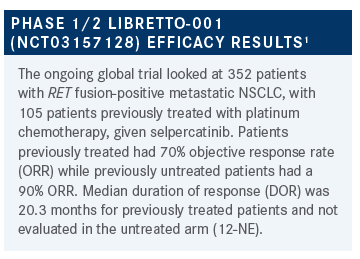
WEISS: I made no attempt to hide my enthusiasm, but by way of disclosure, I helped make this data. In trying to not be a hypocrite, I’ll put that out there. Any reactions, anything surprise you, anything you think worth commenting upon, or asking about?
GILL: I thought it was interesting how, regardless of the PD-L1 percentage, you saw a robust response. In our case, it was tempting, maybe, to think about immunotherapy quickly, but this is a great example of how you really should get all the data first before you jump to a particular treatment. It’s reassuring that, regardless of the PD-L1 status, you know that this would be a great treatment option for this patient.
WEISS: I would underscore that, like most if not all TKIs, selpercatinib does appear to have a few more adverse events [AEs], particularly hypersensitivity reactions in patients with previous PD-1. There are some data out there that PD-1 agents, and PD-L1 agents, are highly unlikely to work in RET-fusion cancer. Between lack of efficacy and increasing the toxicity of what’s really going to help, to really try to avoid immunotherapy in those patients. If there’s really a push to do something, to make that something chemotherapy without immunotherapy. You can always add it to cycle 2, if you get back EGFR, ALK, and RET negative.
MERRICK: As far as I’m aware, I think there are 2 agents that are directed against RET fusion lung cancer. Is there any data, if a patient doesn’t respond to 1, or progresses on 1 agent, whether there’s utility in using the other agent?
WEISS: If you look at the shape of binding, the data on the resistance changes, there’s very little reason to believe that 1 is likely to be active after the other. There are case studies of people reporting that and then get control on the other. On the other hand, when you continue TKI past progression, you can get disease control with this same TKI. My quick answer is that I’m not really doing that in my practice. If the patient is on 1 agent, and I want to treat past progression, I just keep them on the agent I know they’re already tolerating. Probably no harm in switching it up, but I think it’s going to be highly unlikely that you’re going to get a lot of [use] out of that, that that’s going to help a lot.
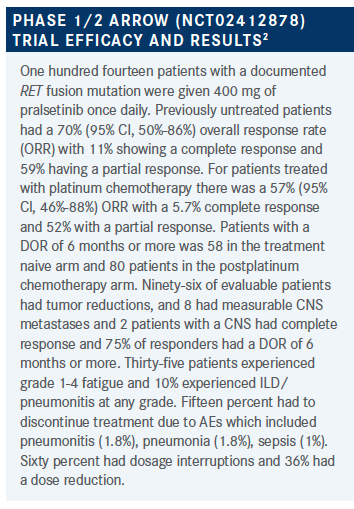
RUDOLPH: [For patients who] did poorly if they had previous immunotherapy in this trial, there were about 45% who had previous immunotherapy?
WEISS: I did say that, in particular with sensitivity type events, with selpercatinib were that seemed to be more pronounced in patients with previous immunotherapy. That’s actually been reported with a number of other non-RET TKIs, as well. To me, the key point is that, like with EGFR and ALK, it should be a first-line drug in RET lung cancer, for both toxicity reasons as well as being both more efficacious and less toxic than chemotherapy. They are also more efficacious and less toxic than first-generation TKIs.
GADDE: I think efficacy-wise, and safety-wise, they seem very similar. With his background, maybe it would be more careful in this population. The patient with rheumatological diseases, you may be a little bit more careful. It sounds like diarrhea was a part of both of them, hypertension was a part of both of them, maybe acute DC would sway you vs the other. I think overall, it’s great benefit in the CNS as well. It sounds like, clearly not to compare across trials, but clearly selpercatinib seems to have had a higher response but I guess that might have swayed it, but again, we can’t compare across trials, technically.
WEISS: Sure, we can. We’re not supposed to, but we all do, so we may as well put in on a table. For formulation, selpercatinib requires 2 pills, depending on weight. Either two 80 mg pills, or an 80 mg and 40 mg pill, and it is twice a day drug. Pralsetinib is more convenient, at 400 mg flat dosing daily, but less convenient in that you have to take it on an empty stomach. As far as AEs, we worry about the QT with selpercatinib, or perhaps we should. I’ll confess that since the trial’s open, I probably don’t get as many EKGs as I should. I haven’t seen torsade de pointes since being an intern in the cardiac ICU. All the trials make us constantly get EKGs and talk about this. With pralsetinib, the conversation is more about ILD and pneumonitis. We should see more tumor lysis syndrome in lung cancer. As far as response rates go, I hesitate to compare across trials, but the numbers are there for your reference, what was reported in these efforts.
RUDOLPH: What are your thoughts about the intracranial response, the differences?
WEISS: Both drugs are predicted to have brain penetration. I joke about not doing cross-trial comparison, and then here I’ve done it. But, when you get down to such small numbers of 9 patients here, I think it becomes very precarious. I’ve disclosed to you already the way in which I may be biased, which is to say I participated in the selpercatinib trial, but what I would say is that it’s better proven with selpercatinib that you have reliable intracranial control. With selpercatinib, we have larger patient numbers, there will be even more in the public realm rather shortly, that really showed that the control in the brain is pretty much as good as the body. I find it a very reliable agent. I suspect we may eventually see similarly favorable data with pralsetinib. For now, my opinion is selpercatinib’s proven it a little better.

GILL: I’d be more scared of ILD, and I think I would really look at it if the patient had a lot of brain disease. Because, like you mentioned, selpercatinib seems to have, at least as of right now. It looks like it’s a very effective drug in that area. So, I would use based upon that.
WEISS: Does either look more convenient, or easier to use, less of a hassle, or is it a wash?
FEAGIN: Well, of course the pralsetinib, the 400 mg once a day on an empty stomach. Once a day is appealing, but the fact of an empty stomach, and timing things, and whether their other medications have to be on an empty stomach, that’s more of a hassle. To speak on the other point, interstitial lung disease is just horrible to see, and there’s so little you can do for it, most times.
GADDE: I think your point was very good in how QT really making that real-world difference. I wonder if further trials can maybe comment on more Torsades or significant events that you would do something about.
WEISS: Yes, this is a sore point for me, because I do a lot of [phase 1 trials] so I spend all day signing off on QTs on EKGs, it’s sort of a pet peeve. I didn’t mean to comment too far on that. We do a lot of that in the trial world. The companies love it, the FDA loves it, and I’m not sure we’re protecting cardiac health all that effectively in our patients on phase 1 when we do it. I don’t think the FDA cares what I think about that.
REFERENCES
1. Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med. 2020;383(9):813-824. doi:10.1056/ NEJMoa2005653 doi: 10.1056/NEJMoa2005653
2. Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22(7):959-969. doi:10.1016/S1470-2045(21)00247-3
