Roundtable Discussion: Borghaei Discusses Combination Therapies in NSCLC With High TMB
During a Targeted Oncology Case-Based Roundtable event, Hossein Borghaei, DO, MS, moderated a discussion about a 59-year-old man with non–small cell lung cancer.
Hossein Borghaei, DO, MS

Speaker list

During a Targeted OncologyTM Case-Based Roundtable event, Hossein Borghaei, DO, MS, a professor and chief of the Division of Thoracic Medical Oncology at the Gloria and Edmund M. Dunn Chair in Thoracic Oncology, codirector of the Immune Monitoring Facility at Fox Chase Cancer Center, moderated a discussion about a 59-year-old man with non–small cell lung cancer (NSCLC).
Case summary
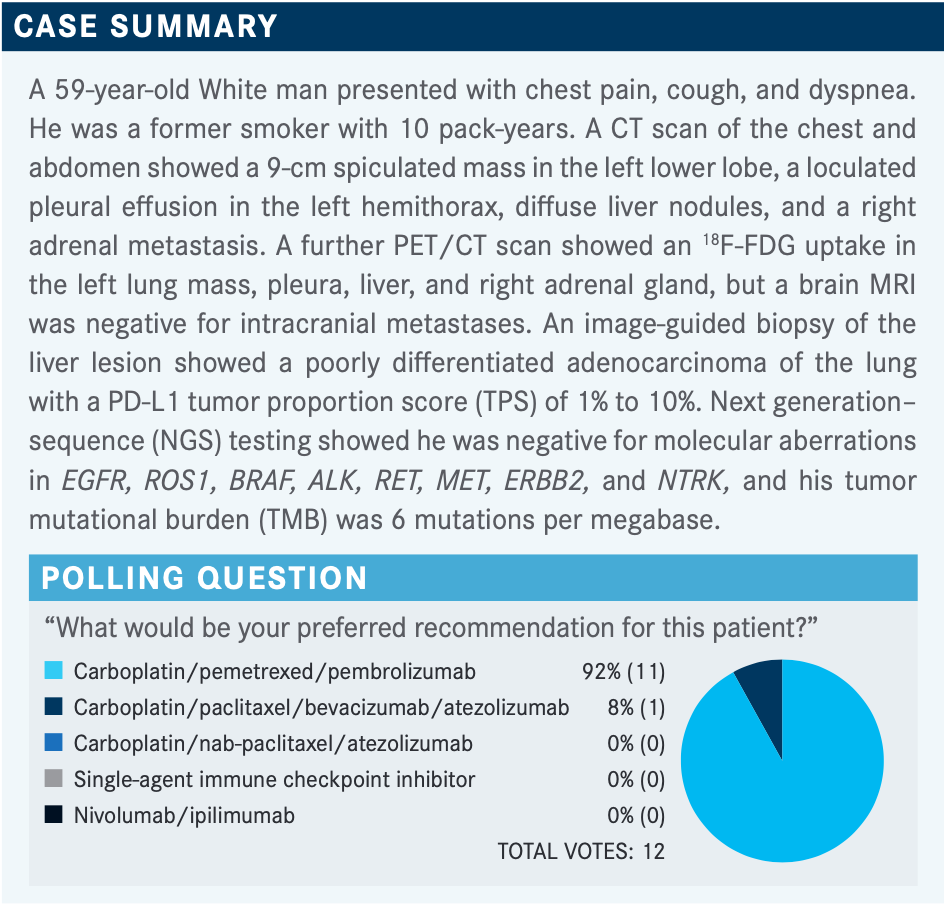
BORGHAEI: Somebody who picked carboplatin, pemetrexed [Alimta], and pembrolizumab [Keytruda], can you tell me what your rationale is for [this combination treatment]?
EFIOM-EKAHA: I think it’s the favored regimen in the folks that don’t have a PD-L1 [TPS] greater than 50%. [It has a] generally well-tolerated and robust response rate with good durable responses, and it was better than chemotherapy alone, so that makes perfect sense. I guess the ipilimumab [Yervoy] and nivolumab [Opdivo] combination would also be reasonable, but I don’t know that it offered me much more. The study [results weren’t] compared with the pembrolizumab and chemotherapy combination, but when I do cross-trial comparison, I know that I can say [the combination with pembrolizumab] offers me much more in terms of benefit....I think right now, for me, pembrolizumab/pemetrexed/carboplatin remains my go-to.
BORGHAEI: Clearly, a lot of our colleagues are agreeing with you because most...voted for that treatment. We got 1 vote for carboplatin/paclitaxel, with bevacizumab [Avastin] and atezolizumab [Tecentriq], and that’s from the IMpower150 study [NCT02366143]. That’s a reasonable option also based on the IMpower150 results; it’s just that paclitaxel is a bit of an older regimen. Most of us would prefer pemetrexed just because of the toxicity profile, not from an efficacy point of view, and I think there’s a huge difference [between those profiles].
It is interesting that ipilimumab/nivolumab got no votes, and I hear you saying you don’t think it’s going to add anything above and beyond what you’re getting with carboplatin/pemetrexed/pembrolizumab and you’re worried about the cost [of the treatment] and toxicity. That sometimes comes into play, although the trials in lung cancer don’t show significant toxicity with the ipilimumab/nivolumab. I would also say, in a very transparent way, that I have always been partial to ipilimumab/nivolumab. I’ve participated in all the studies from phase 1 all the way to phase 3, and the steering committee for CheckMate 227 [NCT02477826]. I clearly have a bias toward an IO [immuno-oncology]-IO regimen based on my own personal understanding that perhaps an IO-IO regimen would be better in combination with chemotherapy. I would also admit that the clinical data suggest that chemotherapy and an IO combination work well.
For someone with a tumor that has a TPS of anywhere from 1% to 10%, I do believe my choice would be [the choice you made] of carboplatin/pemetrexed/pembrolizumab also. I’m in complete agreement there. My preference for ipilimumab/nivolumab is for a different patient population usually. I also think it’s interesting that nobody picked single-agent pembrolizumab, because we have KEYNOTE-042 [NCT02220894], which basically says with the PD-L1 first line you can use that.
If the PD-L1 score was over 50%, would that have changed things for you?
EFIOM-EKAHA: Yes.
BORGHAEI: Do you look at disease burden as an indicator as to whether you want to use chemotherapy or not? With chemotherapy IO, I can see [burden] in the greater-than-50% PD-L1 response rate, even 60% or 70% sometimes. But with single-agent pembrolizumab, does anybody look at liver metastases and see this patient has more disease and [you] want to use a chemotherapy IO? Do you use those clinical criteria?
EFIOM-EKAHA: I tend to do that, even in the high PD-L1 expressors greater than 50%. If you have high-volume disease, especially in the young patient with minimal comorbidities, they can tolerate it. I’ll give some chemotherapy initially. Quite honestly, I do that first scan after 2 cycles, and if they’ve had a nice robust response to the chemotherapy-immunotherapy and maybe a flare of some toxicity, I might back up the chemotherapy and begin the maintenance earlier. But I do think that that response rate will be enhanced even in the higher PD-L1 expressors when I give chemotherapy.
BHANDARI: I was looking at the NCCN [National Comprehensive Cancer Network] guidelines and I was surprised. There was no mention of doublet chemotherapy. If somebody is not able to get immunotherapy, I’m surprised they didn’t mention anything [like that chemotherapy option]. Everything is with the IO or IO-bevacizumab and IO-IO over chemotherapy.
BORGHAEI: Do you see a lot of patients who are not IO eligible?
BHANDARI: Maybe 10%, yes. They didn’t mention it and that’s really surprising.
GOLDBERG: I think you have a choice when the PD-L1 expression is greater than 50% to use monotherapy and obviously you’re still avoiding chemotherapy. On the other hand, with the patient you just presented with pleural effusion and their tumor burden, these viscerally are kind of extreme and you might want to use the combination there. Both treatments have been shown to be effective in [patients with a PD-L1 expression] greater than 50%, and I think the patients who had received chemotherapy and pembrolizumab with greater than 50% did a little bit better. I think you can use both and vary that depending on the age, comorbidity, and visceral crisis. I think the chemotherapy does help progression-free survival [PFS] and that moves it along, because you do get debulking with the immunotherapy.
It’s interesting that we talk about that, but the taxane chemotherapy, carboplatin, and atezolizumab package seemed to work very well for patients who have extensive liver disease. If you’re looking at a clinical scenario, that was a high point of that clinical trial.
BORGHAEI: I’ve seen those graphs suggesting that patients with liver metastases do better with bevacizumab. We sort of know from the older bevacizumab studies that there’s something about VEGF productio [that might support] a rationale there. But honestly, I think I’ve seen data [for patients with] liver metastases on carboplatin, pemetrexed, and pembrolizumab, so I’m not sure if there is that degree of correlation there. I like the fact that you said there are several other factors that go into it, including the clinical parameters that we talked about, like treating existing conditions such as autoimmune disease, and your patient might not be a candidate for that. The other option we didn’t include in the poll is the CheckMate 9LA [NCT03215706] regimen, which is 2 cycles of chemotherapy plus ipilimumab and nivolumab.¹
Polling question
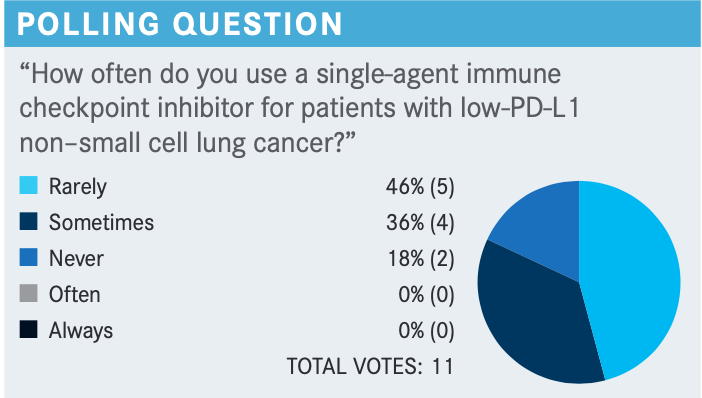
BORGHAEI: In what conditions would you consider using a single-agent inhibitor in a low–PD-L1 patient?
MAMADGI: I voted for sometimes, because going off KEYNOTE-042, if I have an older patient with comorbidities and they have a PD-L1 score above 9, I tend to use a single agent in those cases.
EFIOM-EKAHA: It’s [useful in that] older patient who always has comorbidities and you’re hoping to do something without necessarily hurting them.
FAROUN: I think I use it only in a case when we have renal insufficiency and contraindication for chemotherapy. I think by itself, however, it does not inhibit the use of triplet therapy, in my opinion.
BORGHAEI: Age alone is not a factor, but comorbid conditions might make it difficult for patients to get chemotherapy, [which makes the single agent] a good way of using that.
FAROUN: When the patient comes with pleural effusion, malignant, and you have to use pemetrexed, do you have to do thoracentesis before pemetrexed?
BORGHAEI: The old data say that yes, you should do that, but there is a European study that actually looked at that question. It was smaller, and [the results were] published many, many years ago and suggested that you really don’t have to necessarily do that with peme-trexed. We worry because with methotrexate, we used to get reaccumulation of chemotherapy in the fluids and ascites, and we used to have this condition of having to drain everybody.
With pemetrexed, in the beginning, I used to do that almost every time, but I haven’t really had to go and do a thoracentesis on everybody just because I have some pleural effusion. Does anybody do that?
FAROUN: I do a moderate to large pleural effusion, otherwise [I don’t do a] smaller, loculated one.
ROTKOWITZ: I think I reserve this procedure for an elderly and frail patient. I’ve seen patients do quite well with decent response to therapy. Obviously, I look at KEYNOTE-189 [NCT03950674],and if they’re a candidate for systemic chemotherapy and agreeable to it, I go with the triplet regimen. There are some patients that vehemently refuse chemotherapy or are probably not prime candidates for ipilimumab/nivolumab, and I would offer a single-agent PD-1 inhibitor.
GOLDBERG: Second-line nivolumab is approved [in this patient population] regardless of a PD-L1 expression.
BORGHAEI: I think even pembrolizumab with anything over 1% is approved in the second line. I just haven’t seen too many patients who didn’t get IO in the front line, so I haven’t really treated second line with IO alone in a couple of years since the chemotherapy IO studies came up. You’re absolutely right, though, and we still have the option of using single-agent IO in the second-line setting if the patient didn’t get it.
FAROUN: If the patient has a large cell tumor with a neuroendocrine differentiation, do you use the triplet therapy up front or do you change your chemotherapy?
BORGHAEI: I have used the same regimens that I use for other non–small cell lung cancers [NSCLCs] for my large cell neuroendocrine tumors. The data keep going back and forth, and even on the NCCN guidelines I think it says you can use the same regimen used for NSCLC. As you know, there were not a lot of patients with these kinds of histologies on the clinical trials that we’re talking about, and therefore it’s hard to come up with any data. In the absence of obvious paraneo-plastic syndromes, I don’t really have any problems with using the triplet combination in that patient population in that histology, but definitely [when treating patients with large cell disease] I don’t have any problem with it. With neuroendocrine differentiation there will be a discussion with the pathologist to really see what the extent of that is and then making sure that there is no paraneoplastic syndrome of any kind [before proceeding with this treatment].
PROOTHI: What happens if they have paraneo-plastic syndrome?
BORGHAEI: Well, again, this is an area where we don’t know a whole lot of data, but in the thymic carcinoma role, patients could have a little bit more toxicity with the IO, which is not as well defined, where I can tell if there is a paraneoplastic syndrome. In general, the presence of autoimmune disorders makes me a little bit hesitant. Although as I said earlier, there are studies looking into the use of checkpoint inhibitors in patients with autoimmune diseases. But these are usually well controlled on stable medications and things of that nature. [With] somebody who has an active paraneoplastic syndrome, I might be a little bit hesitant about the use of checkpoint inhibitors, but not [without] a lot of data or without putting somebody on a trial so that we can monitor and learn something from it.
question

FAROUN: In this CheckMate 9LA study, do you have any predictors for the [adverse events (AEs)]? I tried with 1 patient, just 1 dose [of nivolumab/ipilimumab], and she ended up in the hospital with dehydration. To be honest with you, I’m very hesitant to use it. Is there any strategy that can overcome the diarrhea as a preventive, or do you act upon when the patient starts having diarrhea?
BORGHAEI: Not that I’m aware of. I don’t know of anything that’s prophylactically used to reduce the chances of having an AE from the ipilimumab/nivolumab combination. Unfortunately, ipilimumab adds to the toxicity of the regimen, but I don’t know of any way that we can predict who’s going to have more AEs, although that is the subject of a clinical investigation right now looking at germline mutations and genomics. I haven’t seen anything useful yet, and I’m not aware of using anything prophylactically to avoid having immune-related AEs.
EFIOM-EKAHA: One of the things that’s attractive about the CheckMate 9LA [regimen] is that you blunted that initial loss, that progression where the curves will cross. When you look at the ipilimumab/nivolumab vs chemotherapy, you had the initial progressors in ipilimumab/nivolumab and you say, “Gosh, we lost a few patients who progressed early on there.” But when you did use the CheckMate 9LA treatment, you didn’t see that drop again with the addition of the chemotherapy. But again, in my mind I’m trying to see if there is a biomarker to [deter-mine which] patients I should be giving the CheckMate 9LA regimen or maybe just give them ipilimumab/nivolumab compared with KEYNOTE-189.
BORGHAEI: No, and unfortunately that’s the problem. We never developed a biomarker for CTLA-4 to figure out the patients who are really going to get benefit from the CTLA-4 addition to the checkpoint inhibitor. I really don’t have any logical way of choosing someone for ipilimumab/nivolumab or CheckMate 9LA vs chemotherapy-IO. I preferentially like an ipilimumab/nivolumab regimen for the reasons you just said, as it prevents rapid disease progression because of the chemotherapy. However, I use this mostly in patients with PD-L1–negative tumors in my practice simply because I think those patients did well with that regimen in CheckMate 227. Again, we’re talking about 3-year 30% overall survival [OS] rate, which is almost exactly [the same] as the PD-L1 positives. [For] a patient population that doesn’t really have a lot of other options necessarily in a bad sort of a tumor, my argument always was if I use ipilimumab/nivolumab, maybe I can come back to chemotherapy at the time of progression at that point.
But with the CheckMate 9LA preventing that rapid progression with just 2 cycles of chemotherapy, that’s become my favorite regimen to use in the PD-L1–negative [population].1 Obviously, because it’s approved, I don’t have a reimbursement issue the way you might have with the [CheckMate 227 regimen] because [that’s] not approved in the PD-L1 negative population.
REFERENCE
1. Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. doi:10.1016/S1470-2045(20)30641-0

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More