Roundtable Discussion: Addressing Management of Patients with Metastatic CRPC Following Frontline Treatments
During a Targeted Oncology Case-Based Roundtable event, David I. Quinn, MD, PhD, MBBS leads a discussion on treatment for metastatic castration-resistant prostate cancer following frontline treatment.
David I. Quinn, MD, PhD, MBBS

During a Targeted Oncology Case-Based Roundtable event, David I. Quinn, MD, PhD,
MBBS leads a discussion on treatment for metastatic castration-resistant prostate cancer following frontline treatment.
Case Summary
MAR: He has localized high-risk disease, so I probably would have given him [an] extended course of ADT for at least 2 years, but I can’t think of anything else I would have added if there was no evidence up front of any metastatic disease.
DEKKER: I would not have believed that bone scan and CT from the beginning. I would have proceeded with MRI or Axumin scan if I had a question. So this would [be] the only difference I would have early on. If it was confirmed that it is not metastatic disease, then yes, I would have done the same.
VORA: With a PSA that high, [I might have considered] up-front chemotherapy as well. Or, as the previous person had said, I would have looked carefully for a metastatic focus.
QUINN: OK, looking harder—I think that’s good. What’s the evidence base for giving chemotherapy to this patient?
DEKKER: Personal experience [and] there is some evidence. The [National Comprehensive Cancer Network] says that for [a] very high-risk patient, chemotherapy could be considered.1
QUINN: I think that’s reasonable. We had the PUNCH study [NCT00430183], which gave neoadjuvant docetaxel in 6 cycles plus hormone therapy. Although it wasn’t the primary end point, patients who got chemotherapy had an overall survival advantage.2
GROSS: But he’s not metastatic, so I’m highly worried about giving chemotherapy for a nonmetastatic patient. Yet it’s certainly a good idea to look.
QUINN: We were suspicious. We didn’t quite find [that, but] you could do additional imaging. An MRI of the pelvis probably would have found that metastasis. What about a bone-targeted agent? Could they have started that sooner, given that it was localized disease, and he’s getting 18 to 24 months of ADT?
CHARU: It’s reasonable to give a bone-remodeling agent. Not that frequently, but every 4 months or so.
QUINN: So you might consider giving some sort of dose of one of the osteoclast inhibitors to prevent osteoporosis.
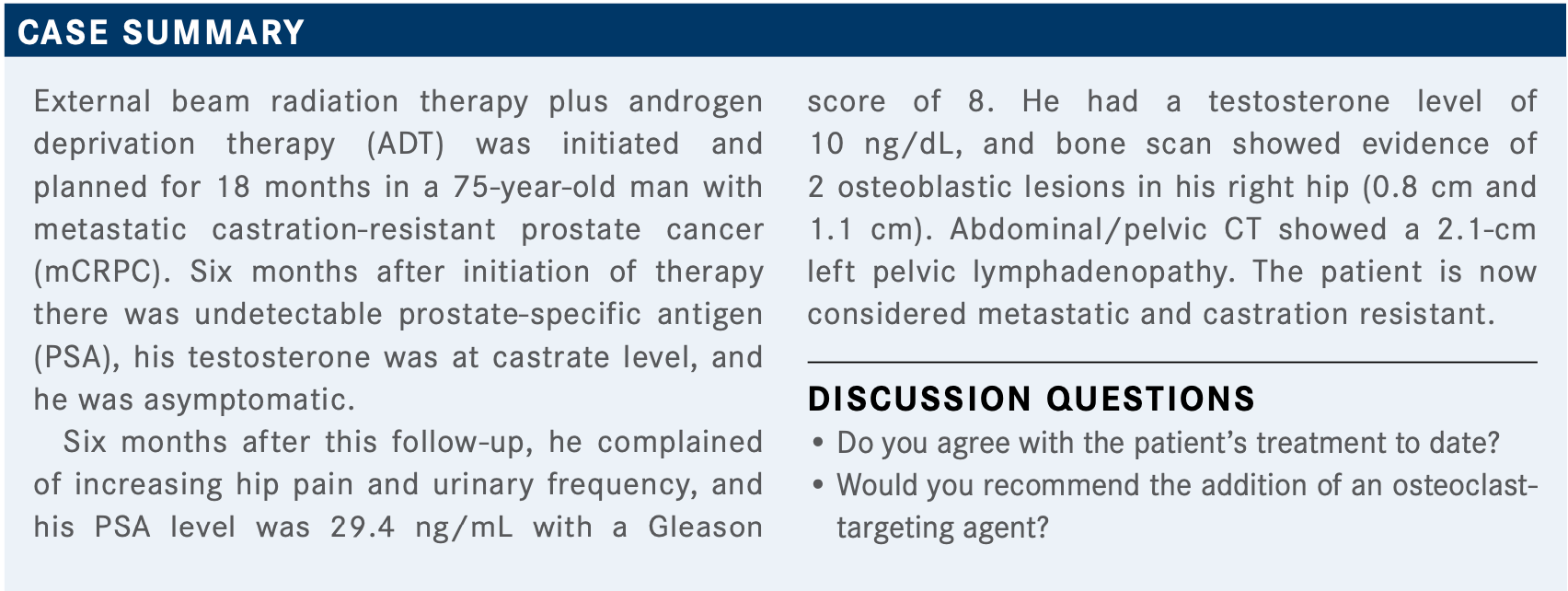
QUINN: How do you pick between these classes of agents? How do you choose between chemotherapy and androgen receptor–targeted therapy in a patient with mCRPC?
DEKKER: I chose docetaxel because the patient had a rather short disease-free interval and had a high Gleason score. I figure the patient will need it sooner or later, so I [had] better give it sooner.
GROSS: Yes, so, there are not great data driving a decision of the first-line agent here, for CRPC. There are suggestions, in retrospective trials, that abiraterone might have longer disease-free intervals in randomized prospective trials, but not powered for survival. If the patients can take abiraterone, I generally try to start with that in the absence of other contraindications.
QUINN: Does anyone want to tell us why they might have picked enzalutamide [Xtandi] over abiraterone [Zytiga] or over chemotherapy?
YEH: If the patient’s highly symptomatic, I think an agent like radium or even chemotherapy is reasonable; that’s what I would do.
If they’re mild to moderate, then both abiraterone and enzalutamide [are acceptable options].
QUINN: The main dictating feature is kind of a pain— maybe the aggression factor in terms of how long the patient has taken to get to this point.
SHIN: Didn’t [the patient have] pelvic lymphadenopathy? Can you use radium-233?
QUINN: Yes, if the lymph node is less than 3 cm in diam- eter, then that would have fulfilled the original trial that was done. The other exclusion criteria are other visceral metastases. You’d have to monitor that lymph node if you’re going to give the patient radium, yes.
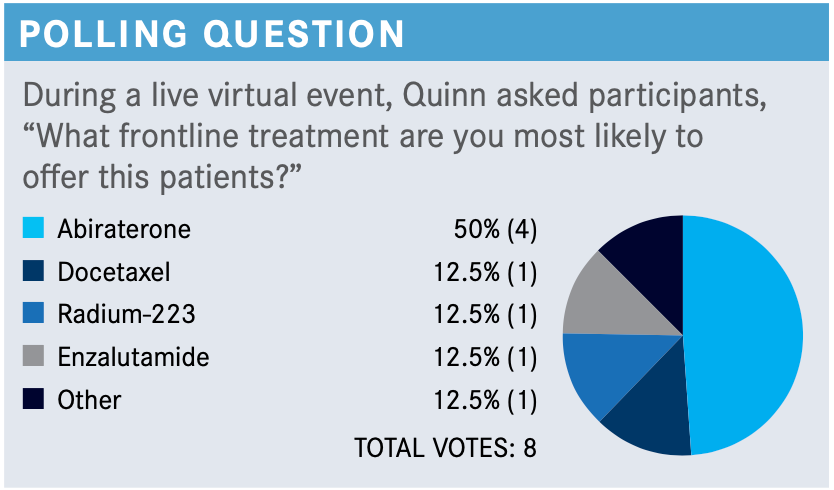
QUINN: [How would you compare] giving cabazitaxel or testing for potential PARP inhibitor usage?
GROSS: The issue is really the potential delay about getting results from DNA-damage response or next- generation sequencing and circulating tumor DNA-based assays. It might be reasonable to do both; it depends on the disease pace. If the [pace of the disease] is very symptomatic, you might have to just [accept the situation] and start treatment, and the best next-line treatment here would be cabazitaxel [Jevtana]. I think that it’s a judgment call. Then you must balance that against [whether the patient] had docetaxel-related neuropathy.
QUINN: We’re going to presume that [the patient is] chemotherapy fit because he had docetaxel before, although the neuropathy’s a problem and it can be a big one. [However, in the polling question,] nobody went for abiraterone after the patient had had enzalutamide. Now the question that comes up is “Would you give cabazitaxel to a patient who didn’t tolerate docetaxel with the neuropathy?”
GROSS: Yes, I think that often you can get away with it. It depends on what the docetaxel-related adverse effect [AE] was. Fatigue and some other [AEs] are much better, and sometimes even taste changes. But if it’s myelosuppression, then you’re probably in for a rough ride.

MAR: Anecdotally, in my practice, I find that cabazitaxel’s a bit lighter to tolerate than docetaxel, for whatever reason. I don’t come across major [toxicities]—maybe blood count issues or diarrhea, and nuisance ones like fatigue or neuropathy in some ways. In general, I don’t think it’s a horribly toxic drug, and I don’t consider the second novel antiandrogen post failure of the first one to even be an option in this setting.
So I don’t really give it as a choice most of the time. If someone failed abiraterone or enzalutamide, I don’t find that the second treatment option works. Very rarely have I had enzalutamide work post abiraterone, and I’ve never had the vice versa work.
QUINN: Are there patients to whom you can’t give cabazitaxel in your practice?
MAR: I have never had one I could not give cabazitaxel to. I can’t think of one off the top of my head.
QUINN: [In your own practices, how many patients are rejecting chemotherapy in favor of oral therapies?]
PHAM: It’s not that big of a deal anymore. I think, with relation to the COVID-19 pandemic, in the beginning patients were much more hesitant to go to the clinic and start chemotherapy. Personally, I’ve seen it shift where patients are much more comfortable now with going to the clinic and any treatment suggestions.
QUINN: Patients are more inclined now to come in and have an infusion, whereas maybe during 2020 that was a problem for some of them.
PHAM: Yes, especially in the beginning of 2020.
DEKKER: I had a little bit of challenge for patients [who had been] worried in April, May, and through June [of 2020], but now it’s not an issue.
VORA: I agree. I think the unknown was what drove most of us to say, ‘Don’t come into the clinic and get your chemotherapy right now until we figure out what our plans are.’ I think after experimenting for 3 to 4 months, we realized that this wasn’t going to end anytime soon, and we needed patients to get their chemotherapy.
QUINN: What about when we got the surge [of new COVID-19 cases] at the end of 2020, early just into 2021? Was there a difference or had [patients] gotten used to it by then?
MAR: I had to tap in mostly with my surveillance and patients [on leuprolide acetate], not the ones who were ill enough to get chemotherapy; those came in regardless of the timing of the COVID-19 case surges. But the ones on surveillance and [leuprolide acetate] who were feeling good, yes, I saw that.
CHARU: In my practice, basically, they don’t come because they’re treating well. [However, patients] who are on active chemotherapy, I think they’re OK and they don’t have any issues coming to the office.
References
1.NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 2.2021. Accessed May 17, 2021. https://bit.ly/3hFCqYX
2.Pietzak EJ, Eastham JA. Neoadjuvant treatment of high-risk, clinically localized prostate cancer prior to radical prostatectomy. Curr Urol Rep. 2016;17(5):37. doi:10.1007/s11934-016-0592-4
