Barata Identifies Options for Nonmetastatic CRPC With Relevant Trial Data
Based on clinical trial data, apalutamide, enzalutamide, and darolutamide are all viable treatment options for nonmetastatic castration resistant prostate cancer.

Based on clinical trial data, apalutamide (Erleada), darolutamide (Nubeqa), and enzalutamide (Xtandi) are all viable treatment options for nonmetastatic castration resistant prostate cancer (CRPC). During a Targeted Oncology Case-Based Roundtable event, Pedro C. Barata, MD, MSc, and assistant professor in Deming Department of Medicine Section of Hematology-Oncolog, Tulan at University School of Medicine, discussed options for the patient population.
Targeted OncologyTM: What are the potential treatment options for this patient with nonmetastatic CRPC?
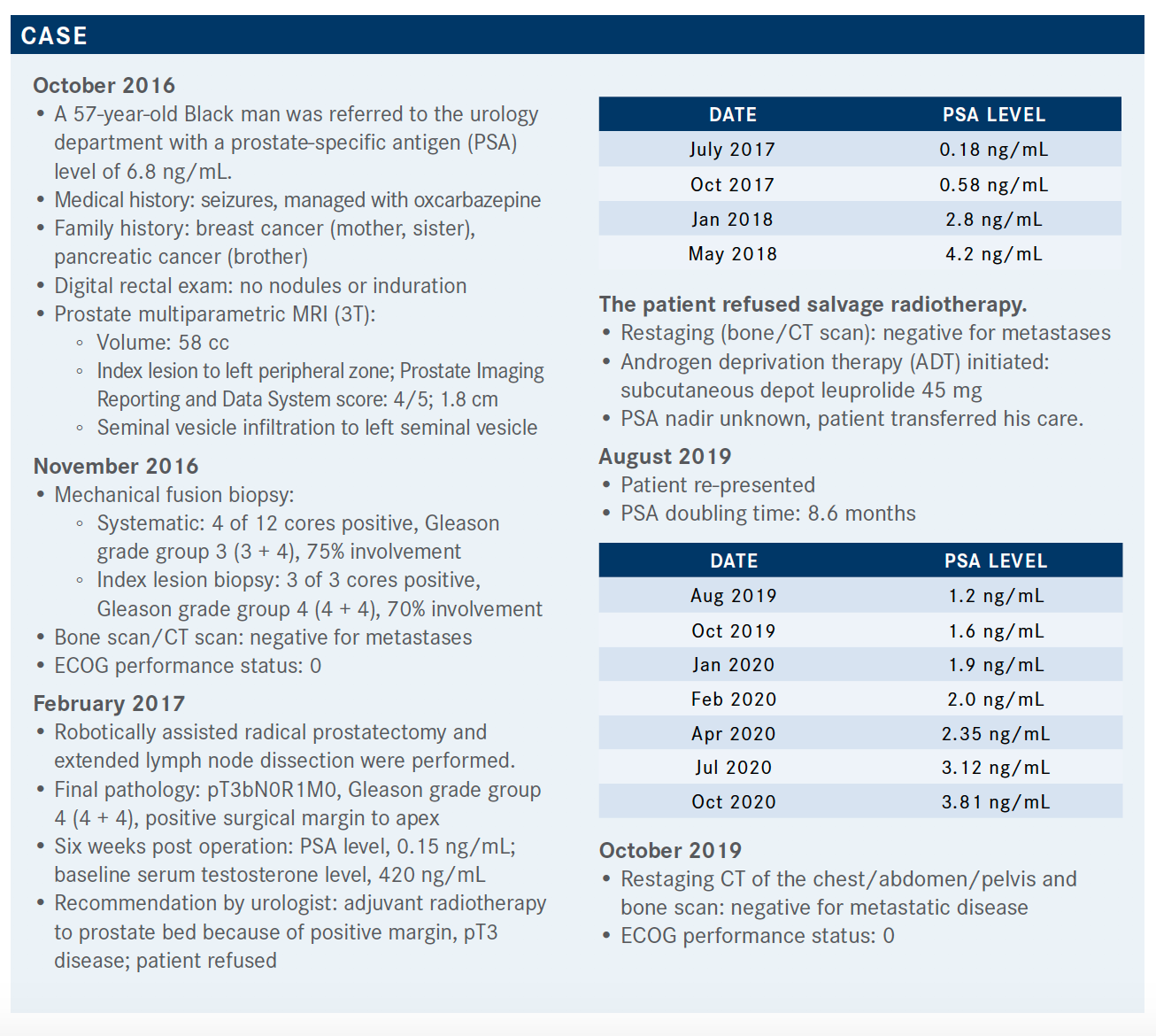
BARATA: The NCCN [National Comprehensive Cancer Network] guideline algorithm recommends looking at the PSA doubling time of patients with CRPC; that is, with PSA progression despite low levels of testosterone.1 For a long PSA doubling time, observation is recommended as there aren’t data that show that initiating treatment prolongs survival. However, for patients with a short PSA doubling time, 10 months or less, there are phase 3 data for 3 category 1 drugs: apalutamide, darolutamide, and enzalutamide. In addition, there are phase 2 data on abiraterone [Zytiga] from the IMAAGEN trial [NCT01314118].2
What evidence supports the use of apalutamide for a patient like the one discussed in the case?
The SPARTAN trial [NCT01946204] was a large phase 3 study that enrolled patients with nonmetastatic CRPC with nodes less than 2 cm below the iliac bifurcation; that is, the study allowed patients with tumors classified as N1 and a PSA doubling time of 10 months or less.3,4 Patients continued receiving hormones and were randomized 2:1. The primary end point was metastasis- free survival [MFS], which was assessed by scans every 4 months until progression. Secondary end points included second progression-free survival [PFS2] and overall survival [OS].
The baseline characteristics of the patients were similar to those of the case study: median age 74 years, about 4.5 months median PSA doubling time, there was a percentage of patients with N1 tumors [about 16%], but most were N0.3 Additionally, about a tenth of patients received bone-sparing agents like bisphosphonate [before the trial]. In terms of the MFS, apalutamide had a 72% risk reduction compared with placebo.3
The median MFS was 16.2 months for placebo compared with 40.5 months for apalutamide, which was significantly different. OS was also significantly improved compared with placebo, with a median OS of 73.9 months compared with 59.9 months and an HR of 0.78 representing a 22% risk reduction.5
In summary, for men with nonmetastatic CRPC, treatment with apalutamide prolongs MFS and OS.3,5
There were no differences in significant adverse effects [AEs; grades 3 and 4].5 However, it is important to note that patients on apalutamide had a longer exposure to the drug [2 years more] than those on placebo as the patients on the placebo group progressed much sooner. The discontinuation rate was around 15% for apalutamide vs 7% for placebo, which is similar to other trials. The most frequently reported AEs for the apalutamide group were fatigue, hypertension, diarrhea, and falls, which are expected.
The rate of falls was 22% for the apalutamide group, 9.5% for the placebo group, and 10% for the placebo to apalutamide crossover group. However, there were 2 unusual AEs worth mentioning.
The first one is a rash, which was seen in about 1 of 4 patients [24%] treated with apalutamide compared with 5.5% with placebo.6 Of the 24%, 5.2% had a grade 3 skin rash and 4% needed systemic steroids. Therefore, it is important to keep this in mind when using apalutamide. The rash led to dose interruptions [28%], dose reductions [12%], or discontinuation [9%]. The second AE worth mentioning is a slight alteration in the thyroid profile [hypothyroidism]. In summary, apalutamide is safe to use in clinical practice.
What evidence supports the use of enzalutamide for a patient like the one discussed in the case?
The PROSPER trial [NCT02003924] had a similar design.7 It was a phase 3 trial that compared enzalutamide with placebo [randomized 2:1] in addition to hormones for men with nonmetastatic or M0 CRPC with a baseline PSA of 2 ng/mL or above and a doubling time of 10 months or less. MFS was the primary end point and there were a number of secondary end points: safety, time to PSA progression, OS, PSA response, and quality of life.
The baseline characteristics were similar to those of the SPARTAN trial. The median age was 74, most patients had an ECOG performance status of 0, the median serum PSA was higher than that in the SPARTAN trial, around 10 ng/mL and 11 ng/mL, with a doubling time of approximately 3.8 months, and around 10% of the patients had received a bone-targeting agent prior to enrollment in the trial.
The median MFS was 36.6 months for enzalutamide compared with 14.7 months for placebo, with an HR of 0.29 representing a 71% risk reduction for progression or death.7,8 In terms of OS, patients treated with enzalutamide lived significantly longer [67 months] than those with placebo [56 months; HR 0.73]. In other words, there was a 27% risk reduction. Therefore, we see the same trend for enzalutamide as for apalutamide: It prolongs MFS and OS compared with placebo.
We have more safety data for enzalutamide compared with apalutamide because it’s been available for longer. Their safety profiles are similar. The overall AE rate was 87% for enzalutamide and 77% for placebo.7 Significant AEs [grade ≥ 3] were the primary reason for treatment discontinuation [9% for enzalutamide and 6% for placebo]. Common AEs such as hypertension and fatigue were reported. There was no difference in the fall rate [grade ≥ 3], which was 1% for both enzalutamide and placebo.
No seizures were reported. In the past, there was a concern regarding the risk for seizures with enzalutamide. Therefore, if a patient has a history of seizures, we avoid the use of enzalutamide. However, the results from big, real-world datasets suggest that there is no increased risk for seizures, which is something to keep in mind. There was also a small, but important number of grade 5 AEs in this trial, 3% with enzalutamide and 1% with placebo.
What evidence supports the use of darolutamide for a patient like the one discussed in the case?
The most recent trial is the ARAMIS trial [NCT02200614], with a design that is very similar to the other 2.9 The study enrolled men with high-risk nonmetastatic CRPC, PSA above 2 ng/mL and doubling time less than 10 months. There were 1500 patients, randomized 2:1 to darolutamide or placebo. The primary end point was MFS and the secondary end points were OS, time to first skeletal symptomatic event, first chemotherapy, time to pain progression, safety, and tolerability.
The baseline characteristics of the patients were very similar to the previous 2 studies: median age, 74, median serum PSA levels of 9.0 ng/mL [with darolutamide] and 9.7 ng/mL [with placebo], and the median PSA doubling time was 4.4 months and 4.7 months, [respectively]. There was a lower percentage of patients with previous use of a bone-sparing agent [3% for darolutamide and 6% for placebo]. Most patients [95% for darolutamide and 96% for placebo] received prior hormonal treatment. There is a subset of patients, in all of these trials, who were treated with a first-generation antiandrogen—bicalutamide, for example.
The median MFS was 40 months for darolutamide compared with 18 months for placebo with an HR of 0.41 representing a 59% risk reduction.9 The OS, at a median follow-up of 29 months, showed a 31% risk reduction [HR, 0.69].10 A median OS was not available because more than half of the patients were still alive. To summarize, the median time for progression on darolutamide is 40 months, while with placebo it is within a year and a half. Although we cannot compare across trials, it’s nice to see the same behavior across the placebo-controlled groups, and [we see a trend where] all these therapies, including darolutamide, more than double the time to disease progression, which is remarkable. Darolutamide is a novel hormonal therapy, and there’s preclinical data suggesting that it does not cross the blood-brain barrier, contrary to what is seen with apalutamide and enzalutamide. Perhaps this is because apalutamide and enzalutamide are more similar to each other than darolutamide.
The difference is all grade AEs are not significant.9 There were less falls [any grade] reported with darolutamide [4.2%] vs placebo [4.7%]. There were similar rates of reports of fatigue and dizziness and a slightly higher rate of a rash. Overall, the safety profile of darolutamide is not that different from placebo. Based on this trial, we cannot say that the safety profile of darolutamide is better than the safety profile for enzalutamide or the safety profile of apalutamide. But in comparison with placebo, darolutamide does not show as much of a difference as the others.
How would you compare the 3 drugs in terms of efficacy and safety?
A side-by-side comparison of the trials [SPARTAN, PROSPER, and ARAMIS] for the 3 FDA-approved drugs [apalutamide, enzalutamide, and darolutamide] for nonmetastatic CRPC shows a common MFS of around 40 months vs about a year and a half for the placebo.11 Therefore, there’s a significant advantage adding these therapies for patients with nonmetastatic CRPC, which translates into an improvement in OS, favoring the addition of antiandrogen.
In terms of safety, we should look at the results from these 3 trials separately and look at the difference between the drug and the placebo. We should highlight that the PROSPER trial showed higher rates of fatigue, hypertension, falls, fractures, and mental impairment with enzalutamide than with placebo.8 For apalutamide, we’re highlighting the rash, falls, and fractures.3,12,13 For darolutamide, there seems to be a smaller difference between the drug and placebo.10
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 2.2021. Accessed March 30, 2021. https://bit.ly/34xiIXZ
2. Ryan CJ, Crawford ED, Shore ND, et al. The IMAAGEN study: effect of abiraterone acetate and prednisone on prostate specific antigen and radiographic disease progression in patients with nonmetastatic castration resistant prostate cancer. J Urol. 2018;200(2):344-352. doi:10.1016/j.juro.2018.03.125
3. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418. doi:10.1056/NEJMoa1715546
4. Small EJ, Saad F, Chowdhury S, et al. Updated analysis of progression free survival with first subsequent therapy (PFS2) and safety in the SPARTAN study of apalutamide (APA) in patients (pts) with high-risk nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2019;37(suppl 7):144. doi:10.1200/JCO.2019.37.7_suppl.144
5. Small EJ, Saad F, Chowdhury S, et al. Final survival results from SPARTAN, a phase III study of apalutamide (APA) versus placebo (PBO) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2020;38(suppl 15):5516. doi:10.1200/JCO.2020.38.15_suppl.5516
6. Erleada (apalutamide). Prescribing information. Janssen; 2018. Accessed May 7, 2021. https://bit.ly/3hgrNvz
7. Hussain M, Fizazi K, Saad F, et al. PROSPER: a phase 3, randomized, doubleblind, placebo (PBO)-controlled study of enzalutamide (ENZA) in men with nonmetastatic castration-resistant prostate cancer (M0 CRPC). J Clin Oncol. 2018;36 (suppl 6):3. doi:10.1200/JCO.2018.36.6_suppl.3
8. Sternberg CN, Fizazi K, Saad F, et al; PROSPER Investigators. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206. doi:10.1056/NEJMoa2003892
9. Fizazi K, Shore N, Tammela TL, et al; ARAMIS Investigators. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246. doi:10.1056/NEJMoa1815671
10. Fizazi K, Shore N, Tammela TL, et al; ARAMIS Investigators. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040-1049. doi:10.1056/NEJMoa2001342
11. Gupta R, Sheng IY, Barata PC, Garcia JA. Non-metastatic castration-resistant prostate cancer: Current status and future directions. Expert Rev Anticancer Ther. 2020;20(6):513-522. doi:10.1080/14737140.2020.1772759
12. Small EJ, Saad F, Chowdhury S, et al. Apalutamide and overall survival in nonmetastatic castration-resistant prostate cancer. Ann Oncol. 2019;30(11):1813-1820. doi:10.1093/annonc/mdz397
13. Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79(1):150-158. doi:10.1016/j.eururo.2020.08.011

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More