Powell Reviews Updated IO/TKI Data and AE Management in Endometrial Cancer
During a Case-Based Roundtable® event, Matthew A. Powell, MD, discussed the case of a patient with advanced endometrial cancer treated with lenvatinib plus pembrolizumab who experienced grade 2 treatment-related hypertension.
Matthew A. Powell, MD
Professor of Obstetrics and Gynecology
Chief, Division of Gynecologic Oncology
Washington University School of Medicine
Siteman Cancer Center
St Louis, MO

CASE SUMMARY
- A 71-year-old postmenopausal woman presented with abnormal uterine bleeding, increasing urinary frequency, and nausea/cramping for approximately 6 months.
- She had 2 grown children and no known family history of cancer.
- Her body mass index was 32; she had type 1 diabetes since childhood, well controlled with medication.
- Physical examination was notable for enlarged uterus and right lower quadrant abdominal tenderness on palpation.
- ECOG performance status: 1
- CT of chest, abdomen, and pelvis showed uterine and bladder masses.
- Cancer antigen (CA) 125 level: 38.6 U/mL
- Endometrial biopsy: endometroid adenocarcinoma, International Federation of Gynecology and Obstetrics stage IVA, grade 3 (poorly differentiated)
- Notable molecular features: mismatch repair proficient (pMMR), microsatellite stable, estrogen receptor negative, HER2 negative, NTRK negative
- Germline testing showed no pathogenic variants.
- Carboplatin/paclitaxel chemotherapy was initiated; treatment was well tolerated other than grade 1 peripheral neuropathy and vomiting.
- Four months after initiation, she had no evidence of disease on PET scan, with complete resolution of symptoms.
- Twelve months post chemotherapy, rising CA 125 level was documented.
- CT scan of the abdomen and pelvis showed growth of bladder mass and new suspicious peritoneal lymph nodes.
- PET scan: intense fluorodeoxyglucose F 18–avid lesions in lungs and peritoneum, as well as para-aortic lymph nodes
- Interventional radiology biopsy revealed recurrent endometrial carcinoma.
CASE UPDATE
- The patient received lenvatinib (Lenvima) at 20 mg daily plus pembrolizumab (Keytruda) 200 mg every 3 weeks as second-line therapy.
- At 3-month follow-up, the patient exhibited a good partial response but complained of occasional morning headache. Her vital signs showed hypertension compared with baseline but electrocardiogram (ECG) showed no abnormalities. Her abdomen and pelvis were nontender with no evidence of palpable masses. The physician’s impression was grade 2 VEGF receptor inhibitor– associated hypertension.
Peers & Perspectives in Oncology: What are your impressions of this patient’s hypertension, and how can it be managed?
POWELL: When we think about VEGF inhibitors…we know how much [VEGF] interacts with our endothelium system, and hypertension is a big part of the management of them. [Lenvatinib] is certainly no exception. These hypertensive issues can happen early, they can happen quickly, and they can be quite profound. They can happen like in this patient at 3 months with slowly increasing blood pressure over time, leading to some adverse events such as headaches. There are good guidelines for introducing therapies to treat this. We will often have patients who have borderline hypertension or hypertension prior to starting therapy get on antihypertensives to help aid with this. For a lot of these patients, we’ll have them checking their blood pressure daily until it’s under good control, and then every other day and then [less frequently], while reporting to us the factors associated with it. [Using] some pretreatment and working with these patients to manage their hypertension is critical. This is something [we] deal with frequently, and it can be difficult for patients because they can have pretty profound blood pressure elevations, especially at that 20-mg dose.
How do you follow a treatment algorithm for onco-hypertension?
I think many [oncologists] have their agents of choice, whether that’s an ACE [angiotensin-converting enzyme] inhibitor or ARB [angiotensin receptor blocker], or a calcium channel blocker. All have been reported and as far as what’s best to use or preferred for patients with diabetes, certainly we tend to [favor] the ACE inhibitors or ARBs.1 There are not a lot of data within cancer specifically, when we have a VEGF inhibitor drug, to know the right agent for each group of patients, but I think, again, as is commonly thought of that, it will be choosing this category. Older patients and those with isolated systolic hypertension tend to do a little better with a calcium channel blocker. We do tend to avoid diuretics unless other agents have been tried.
What mitigation strategies are there for lenvatinib-associated hypertension?
When we think about treating these patients, [the strategy] is coming from the Study 309/KEYNOTE-775 clinical trial [NCT03517449].2 This was the trial comparing lenvatinib/pembrolizumab…with chemotherapy. Looking at the grades of hypertension and the dose reduction strategy that was utilized, typically for patients who had grade 3 hypertension, so over 160/100 mm Hg, these patients [had lenvatinib] withheld until we got their blood pressure under better control, down to grade 1 or 0. If they get to grade 4 with hypertensive crisis, they are typically not going back on therapy. The tricky part is if you don’t have them well controlled and you start them at 20 mg, you could get to grade 4 pretty quickly with some very high blood pressure. I encourage physicians to get patients’ blood pressure under good control prior to starting. Be very quick to dose hold or dose delay when we start these agents.
CASE UPDATE
- Antihypertensive monotherapy was initiated with valsartan, 160 mg daily.
- Patient returned to office for blood pressure monitoring every other day in week 1, then twice weekly for 28 days.
- At 30-day follow-up, she reported resolution of morning headaches.
- Blood pressure was 120/84 mm Hg (hypertensive) vs baseline of 118/76 mm Hg; heart rate of 72 bpm and regular. ECG showed normal sinus rhythm. Hypertension improved from grade 2 to grade 1.
- The patient was to continue oral ARB with a blood pressure check every 2 weeks.
- She would continue lenvatinib at 20 mg daily plus pembrolizumab 200 mg every 3 weeks and monitor for continued response to therapy.
What was the design of the phase 3 Study 309/KEYNOTE-775 trial?
[The trial was investigating] the lenvatinib/pembrolizumab combination vs doxorubicin or paclitaxel.3 The doxorubicin was dosed at 60 mg/m2 every 3 weeks or paclitaxel 80 mg/m2 per week for 3 weeks on, 1 week off. These patients all had measurable disease by blinded independent central review [BICR]. They had to have 1 prior platinum-based chemotherapy, have good performance status, and have tissue available for MMR testing. These patients were stratified based on their MMR status, region, their ECOG performance status, and whether they had pelvic radiation. The primary end point was progression-free survival [PFS] by BICR. Patients had a maximum cumulative dose of doxorubicin at 500 mg/m2.
The patients were well matched [and were] fairly typical for patients needing systemic therapy. They’re in their mid-60s, and [approximately 60%] had traditional endometrioid carcinoma [and over] 40% had prior history of radiation. When this trial was done, we were probably using radiation more than we are now. We see that rate coming down a little bit as we’re backing off on some of the radiation based on some other trials that have been done. High-grade and serous carcinomas are almost half of what we’ll see, with clear-cell carcinoma and mixed tumors [included] as well.
What were the outcomes for patients on this trial with longer follow-up?
In the pMMR population, there was improvement of [nearly] 6 months in median overall survival with the lenvatinib/pembrolizumab combination over chemotherapy. In the all-comers population, it was approximately 7 months.2 There was a 30% improvement in the hazard of death with the utilization of lenvatinib plus pembrolizumab [in the pMMR group (HR, 0.70; 95% CI, 0.56-0.83)]. We’re starting to see some tails at the end of the curves flattening out, so some of these patients are doing well for a long time when we start to look at over 3 years of follow-up. Keep in mind that some of these patients receiving chemotherapy crossed over.
The data [showed] doubling of the PFS for both the pMMR population and the overall population, with a 44% improvement in PFS [in the all-comers population (HR, 0.56; 95% CI, 0.48-0.66)]. These patients are not doing well with chemotherapy, with 3.8 months median PFS in [both] the pMMR population and the all-comers population. They are doing better with the lenvatinib/pembrolizumab combination [6.7 months in pMMR, 7.3 months in the all-comers population]. The key here is if they progressed on lenvatinib/pembrolizumab, we still have the chemotherapy options available, and vice versa. We will typically end up using these chemotherapy regimens in third- and fourth-line therapy.
What do the response rates in this trial show?
Looking just across the response rates, it is a doubling of everything. The response rate in the pMMR population goes from 15% [with chemotherapy] to 30% [with the study regimen], and it is a little more than doubled when you look at all patients.3 The deficient MMR population was a small cohort of 65 patients in each group showing that improvement as well, when you have the addition of checkpoint inhibition for these patients. The median duration of response was [9.2] months in the pMMR population with lenvatinib/pembrolizumab and [14.4] months for the all-comers population. The disease control rate also goes up dramatically to 71.7% in the pMMR population. Patients are feeling benefit even if they’re not getting a response. The time to response is relatively quick at 2 months, so many patients get fairly quick positive feedback on their first scan.
In the intent-to-treat population, subsequent therapy was given to 28% of those in the lenvatinib/pembrolizumab group [and 48.1% in the chemotherapy group]. And 9% of the pMMR patients in the chemotherapy group received subsequent lenvatinib/pembrolizumab. In the deficient MMR population, 17% received a PD-1 pathway drug for additional therapy. This was not as much use as you’d see now because these weren’t available when this trial was done.
What toxicities were observed in the Study 309/KEYNOTE-775 trial?
When we look at adverse events [AEs] of any cause that [occurred] in more than 25% of patients, the biggest AEs are hypertension and hypothyroidism.3 The grade 3 hypertension [rate was 37.9% with lenvatinib and pembrolizumab]. That’s why it’s so important to think about aggressive early treatment to avoid those grade 3 AEs.… Diarrhea is another one we deal with because we’re always worried as we give checkpoint inhibitors and a tyrosine kinase inhibitor [TKI], [because] both can cause diarrhea. Typically, if it happens early, it’s probably the TKI, [but if it’s] happening later, more likely it is the [immunotherapy], but it can still be the TKI. The nice thing about an oral drug is that we can stop it; if the diarrhea gets better quickly, that’s usually related to [the TKI] and we can just monitor with a dose hold, dose delay, and antidiarrheals, but we need to be cognizant of immune-related colitis. But chemotherapy has AEs as well. Dose reductions due to adverse events are very common, and two-thirds of the patients had a dose reduction of lenvatinib as part of this trial.
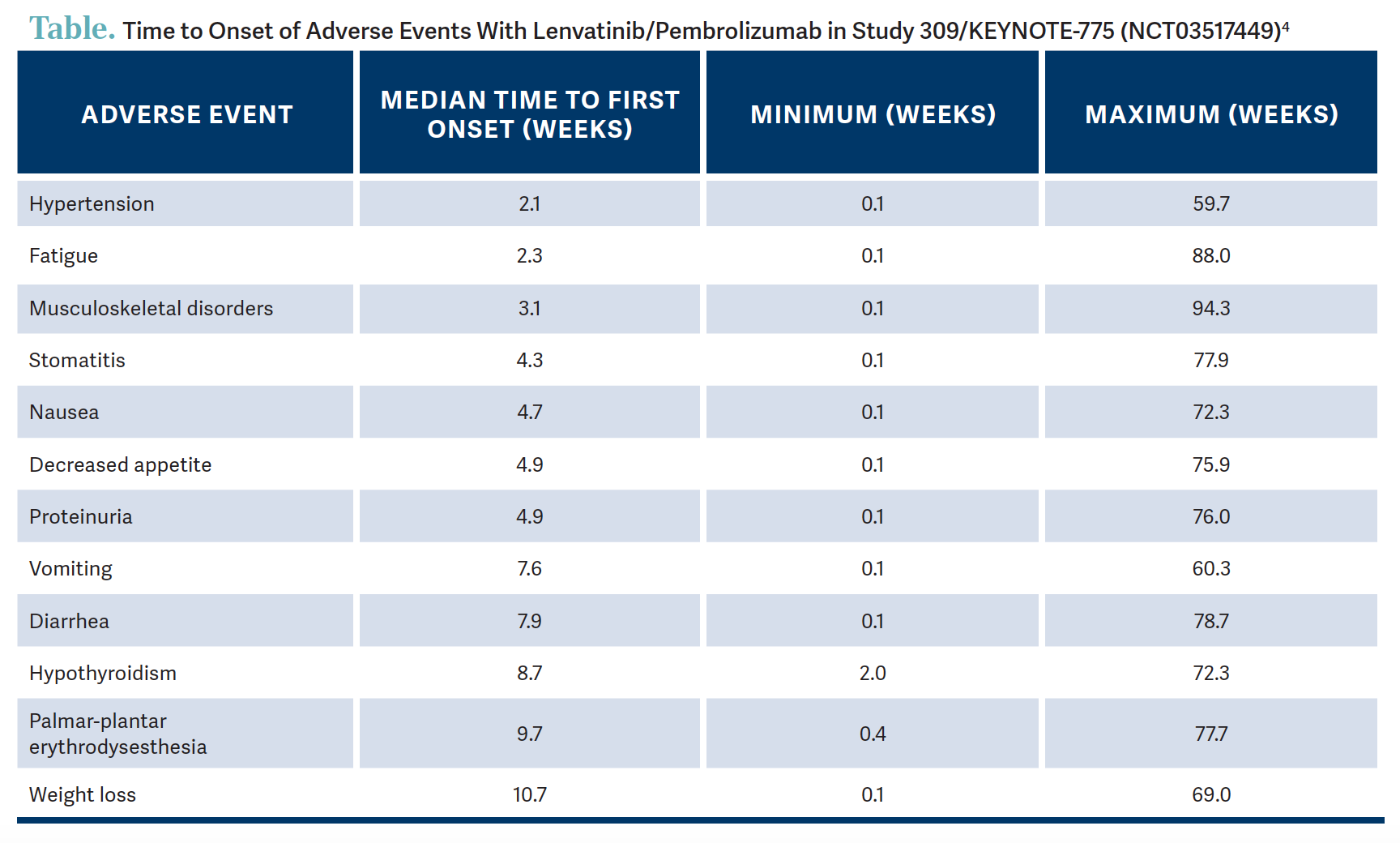
How long after treatment can various AEs be expected to occur?
There is a very nice diagram that Nicoletta Colombo, MD, PhD, published in The Oncologist in 2023.4 For those using lenvatinib/pembrolizumab, it’s a very nice resource showing that hypertension happens quickly [Table4]. Fatigue also is a big issue; joint aches and pain and other musculoskeletal disorders often will tend to get better with time as the patients get on their other doses. But decreased appetite and weight loss tend to persist, and there is some proteinuria. Vomiting tends to happen a little bit later but often can be well controlled in those patients who get nausea. [And] 34% of patients had weight loss. Palmar-plantar erythrodysesthesia syndrome can happen [21.9%, any grade]. It can happen with all chemotherapy drugs, but it was grade 3 [or 4] in 2.7% of patients on this trial, so that is something to keep track of.
REFERENCES:
1. Mohammed T, Singh M, Tiu JG, Kim AS. Etiology and management of hypertension in patients with cancer. Cardiooncology. 2021;7(1):14. doi:10.1186/s40959-021-00101-2
2. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab in previously treated advanced endometrial cancer: updated efficacy and safety from the randomized phase III Study 309/ KEYNOTE-775. J Clin Oncol. 2023;41(16):2904-2910. doi:10.1200/JCO.22.02152
3. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437-448. doi:10.1056/NEJMoa2108330
4. Colombo N, Lorusso D, Monk BJ, et al. Characterization and management of adverse reactions in patients with advanced endometrial cancer receiving lenvatinib plus pembrolizumab. Oncologist. 2024;29(1):25-35. doi:10.1093/oncolo/oyad201

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More