Peers Discuss Role of Pola-R-CHP vs R-CHOP in Newly Diagnosed DLBCL
During a Case-Based Roundtable® event, Haifaa Abdulhaq, MD discussed with participants whether the POLARIX trial influences their choice to use the pola-R-CHP as opposed to R-CHOP regimen for patients with newly diagnosed diffuse large B-cell lymphoma.
Haifaa Abdulhaq, MD (MODERATOR)
Clinical Professor of Medicine
Director of Hematology
University of California San Francisco-Fresno
Fresno, CA

EVENT REGION: California
PARTICIPANT LIST Andrea Edwards, MD | Draupadi Talreja, MD | Rajesh Behl, MD | Swarna Chanduri, MD | Albert Dekker, MD | Xinting Fu, MD | Merin Stephen, MD
ABDULHAQ: For patients who have stage II with extensive mesenteric disease or stage III to IV DLBCL, the preferred regimens are either R-CHOP [rituximab (Rituxan), cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate (Oncovin), and prednisone], which is category 1, or pola-R-CHP [polatuzumab vedotin (Polivy), rituximab, cyclophosphamide, doxorubicin hydrochloride, and prednisone] for patients who have an IPI score of 2 or higher, like our patient. R-EPOCH [rituximab, etoposide phosphate, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride, and prednisone] is also recommended, but it is not considered category 1.1
DISCUSSION QUESTIONS
- For patients with newly diagnosed high-intermediate risk DLBCL: What is your treatment of choice?
- Does it vary based on patient factors?
- Does transplant eligibility affect your choice?
EDWARDS: I often go with R-CHOP. It works very well. Pola-R-CHP also is intriguing based on recent information. But a lot of times for straightforward cases, you can get good responses just with R-CHOP.
TALREJA: My choice has been R-CHOP for 20 years, and I’ve had amazing responses. Patients with stage IV disease go into remission for 5-plus years, and polatuzumab will be more toxic, so I’m not going to use polatuzumab…. I usually save R-EPOCH for patients with double-hit lymphoma; I don’t [use it for those with] large cell or intermediate [risk]. I’m very happy with R-CHOP. I could switch to polatuzumab, but I think it’s a little more toxic.
ABDULHAQ: Does your choice vary based on patient factors? Do you take into account the IPI to decide whether to go with R-CHOP or pola-R-CHP, or any other factors?
TALREJA: [I would use it] more in younger and more fit patients who do not have any issues. This patient is not too old [and] he’s basically healthy, so he could qualify, but I don’t think you’re going to get a higher cure rate compared with R-CHOP. R-CHOP is not as expensive as polatuzumab, and I will use polatuzumab when I’m bridging the patient for stem cell transplant or CAR [chimeric antigen receptor] T-cell therapy, not routine standard of care.
BEHL: I use R-CHOP for the most part. I think pola-R-CHP is very well tolerated. The only [cases in which] I tend to use that is the ABC [activated B-cell] subtype, because I didn’t see that much advantage in others. I pretty much stopped using R-EPOCH because all the data now show whether it’s double-hit or not; I don’t think the R-EPOCH is any better than R-CHOP.2,3 For the most part, I use R-CHOP. I find that sometimes in an older patient with an ABC subtype, I may prefer pola-R-CHP. It is tolerated a little better. My only problem is it’s very expensive…but there have been some data suggesting that the progression-free survival [PFS] benefit might override the cost in certain circumstances.4 In a very selective [population of] patients with ABC subtype [who] are older, I prefer to use pola-R-CHP. But for the most part, [I still use] R-CHOP.
CHANDURI: I use pola-R-CHP because I found that the toxicity is much less and it’s well tolerated. I used to use R-CHOP, but most of my patients have tolerated [polatuzumab] so I have switched to pola-R-CHP.
DEKKER: I tried it in 3 patients. Pola-R-CHP did not work very well. Maybe those were too high-risk patients or there may be other factors.
ABDULHAQ: Was that an issue with efficacy or did you encounter toxicity?
DEKKER: It was both; the patients had adverse events [AEs], and 2 patients were [essentially] primary refractory.
ABDULHAQ: [Did] these patients have high IPI scores? Did they have other risk factors?
DEKKER: Yes, these [had] high IPI scores but again, if you’re looking for patients who have a favorable profile, [any of the treatments could] work.
ABDULHAQ: That’s right, and pola-R-CHP [trial results showed] higher PFS for patients who had an IPI score of 2 or higher.5
CASE UPDATE
The patient was started on pola-R-CHP.
DISCUSSION QUESTIONS
- Would you agree with the choice of pola-R-CHP for this patient?
- What efficacy and/or safety data support your choice?
- Would your treatment plan change if the patient was older than 70 years?
- Would it change if the patient was frail or had poor performance status (eg, ECOG performance status of 1 )?
- If the patient’s subtype was ABC or GCB (germinal center B), would that change your decision?
ABDULHAQ: Based on the data presented from the POLARIX study [NCT03274492]…do you agree with the choice of pola-R-CHP for this patient? And which data support your choice?
TALREJA: [I would ask,] “What is his insurance status? What insurance does he have?” Because I don’t want a patient…telling me they can’t afford 20% [co-pays], and things like that. R-CHOP is pretty [inexpensive]. Polatuzumab is not. [Performance status] and subtype don’t make any difference. They are equal, a little more efficacious with polatuzumab in PFS [but overall survival (OS)] was the same [From the Data5]. If a patient has Medicare, and Medicare doesn’t pay us, HMOs [health maintenance organizations] complain. I would base it on what his insurance status is.
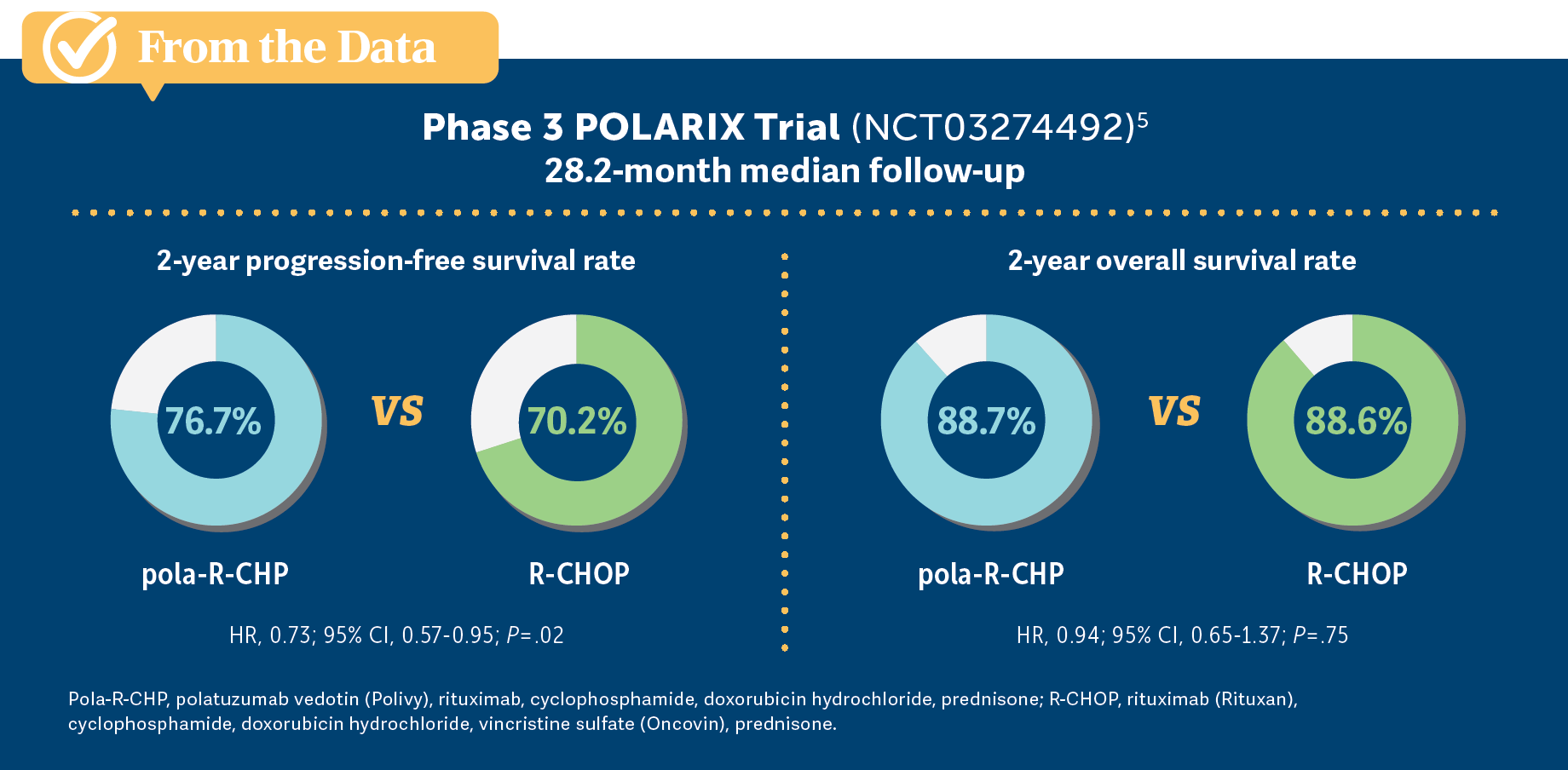
ABDULHAQ: What about the PFS at 24 months? Do you consider that an important milestone in treating your patients?
TALREJA: Yes, it is, but then again, I’m pretty biased with my [positive] R-CHOP experience for all these 20 years.
ABDULHAQ: Your points about insurance and the higher price are well-taken, although [Dr Behl] pointed to a study that was published in Blood that looked at the cost-effectiveness of pola-R-CHP in first-line treatment of DLBCL. That study concluded that using this regimen is cost-effective because these patients have higher PFS, so you end up using fewer subsequent lines of therapy and less CAR T-cell therapy.4
TALREJA: If a patient had bulky disease and I was aiming for a cure, or they were going for a stem cell transplantation or CAR T-cell therapy, I might favor pola-R-CHP more. But if that was not a factor, I would say I’m happy with R-CHOP.
BEHL: I reserve [pola-R-CHP] for patients with ABC subtypes and patients with poor performance status. Now that there is a category 1 recommendation, I don’t find insurance companies have issues with paying for it. It’s fairly well tolerated. But I find it expensive and unnecessary to give for most patients with the GCB subtype. Given that we have long-term data and we only have PFS and not OS data on advantage with this particular regimen, I am reserving it mostly for patients with the ABC subtype.
ABDULHAQ: What you’re pointing to is the subgroup analysis from the POLARIX trial, which did not show added benefit in the patients with the GCB subtype.5 It was an unplanned subgroup analysis, and I’m not sure what conclusions we can [draw] from that, but that showed that most of the benefit was in the patients with ABC subtype. There was a significant percentage of patients who had unclassified lymphoma. I’m not sure how much that affects the subgroup analysis.
DEKKER: [Pola-R-CHP] is not a wrong choice. It’s one of the regimens to consider. Ultimately, it will be the decision of the physician and the patient, and maybe the [health system], on how to approach the situation. If somebody went with pola-R-CHP, that would be appropriate. If somebody decides not to go with pola-R-CHP, it’s also a reasonable approach.
ABDULHAQ: So would you use pola-R-CHP for patients who have poorer performance status vs the patients who have better performance status? Does that affect your decision at all?
FU: That’s a good question. We often have a patient [who is] 82 years old who doesn’t have bad performance status, but it’s not great either. Often I do R-mini-CHOP [reduced-dose CHOP], but I’m wondering: Is this an option [with polatuzumab]? Compared with mini-CHOP, is this comparable in terms of AEs?
ABDULHAQ: I don’t think we can tell because we don’t have a study that compared R-mini-CHOP vs polatuzumab plus R-mini-CHP. I’ve used it on some of my older patients. I’ve used polatuzumab plus R-mini-CHP, but that would be considered off-label use. I am not aware of studies looking at that.
FU: The good thing about R-mini-CHOP is—besides the cost issue—with the R-mini-CHOP you can do a test run, and if the patient will handle it well, you can keep increasing the dose to the standard dose. More than half the time I can go to the standard dose.
ABDULHAQ: So you start with the lower dose and then you build up, which we sometimes do with our older patients. I’ve done that, but I have to say it’s off label. I don’t think we have data on that yet with a polatuzumab regimen.
DISCUSSION QUESTION
- When choosing among the first-line options, what patient factors impact your choice?
ABDULHAQ: After the data presented on R-CHOP, dose-adjusted R-EPOCH, and POLARIX, what efficacy and safety data are the most important for you?
TALREJA: For me, it’s always OS. That’s what I look for in any drug combination: Where is my overall survival going to take me? Then, of course, the PFS and…[other efficacy factors]. Also, financials are very important.
FU: The efficacy data are easier to determine, but the safety data are more difficult. The discontinuation rate may be more reliable in terms of AEs.
CHANDURI: I also look at the safety data. [For] all of the patients, we’ll get G-CSF [granulocyte colony-stimulating factor], even for R-CHOP. For some of the patients, we have to give them G-CSF to give them adequate doses and complete [the regimen]. If their performance status is poor, and if they’re not candidates for R-CHOP, they’re not candidates for pola-R-CHP, either. They are the ones who we try with R-mini-CHOP, but if they are equally young and fit, I think [pola-R-CHP] is the better regimen, and that’s the reason I switched to pola-R-CHP. I’ve had a couple of patients [ for whom] it was successful so far. I haven’t had to send them for CAR T-cell therapy yet.
ABDULHAQ: Got it, and that’s honestly one of our goals. As oncologists, OS has always been our most important primary end point. But in a disease like DLBCL, it’s also important not to have the patient on second- or third-line treatment, because then there’s more cost and more toxicity to the patient.
CHANDURI: Most insurances now approve it, even Medi-Cal [Medicaid in California]. I had a patient with Medi-Cal, and it was approved. We have to fight and maybe appeal, but insurance [companies] are paying. Only with Medicare, if they don’t have secondary insurance, those are the ones [where] even for rituximab in R-CHOP, it’s expensive, and they have to pay more money. I think all insurances are picking up [polatuzumab]. HMOs are paying for this.
ABDULHAQ: Right, because if a regimen is FDA approved, and it’s in the NCCN [National Comprehensive Cancer Network] guidelines…
CHANDURI: As a category 1 [recommendation], they all will pay.
ABDULHAQ: Yes, then it’s hard for insurances to deny it.
EDWARDS: I’m looking at efficacy data because lymphoma is one of those cancers that even in advanced age is potentially curable. It’s very rare for us to say that a patient has stage IV cancer and we can potentially cure it, so efficacy is important [to me] in lymphoma. I want a regimen that has the potential for remission so that the patient can have a better OS. We have supportive care where we can predict how to manage the safety issues to support the patient through efficacious therapy.
DEKKER: A complete response [CR] is a surrogate [end point] because CR is a surrogate of remission. We know that remission may not be durable, but if you have not achieved remission up front, you will probably not achieve remission later.
BEHL: I’m not going to change my practice except in the setting of the ABC subtype. I’m not convinced that in the end this is all that much better in the general population. We have a lot of experience with R-CHOP. If it is an ABC subtype, even though it was not a planned subgroup analysis, we only have the PFS data right now. The CR rate is not that different in both arms; it’s just that the PFS rates are different.
ABDULHAQ: That’s right. The CR at the end of the treatment was similar between both arms. It’s the PFS and the event-free survival that were better with pola-R-CHP.5
STEPHEN: I haven’t been using pola-R-CHP; I’ve been doing R-CHOP, but I do find the data like the PFS to be valuable. I’m thinking [it could be used not only] for the younger patients with more aggressive disease, but the older patients [because] when you [treat] them with the second line they can deteriorate quickly when the disease relapses. That PFS extension becomes valuable, even in those older patients. I’m going to try to figure out in whom to incorporate it more if those data are applicable.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 1.2024. Accessed February 20, 2024. http://tinyurl.com/4tnnkr68
2. Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: clinical outcomes of the phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol. 2019;37(21):1790-1799. doi:10.1200/JCO.18.01994
3. Magnusson T, Narkhede M, Mehta A, Goyal G. No difference in overall survival between R-CHOP and R-EPOCH among patients with advanced stage MYC-rearranged, double hit, or triple hit diffuse large B-cell lymphoma. Presented at: 2021 European Hematology Association Congress; June 9-17, 2021; virtual. Abstract S224. Accessed February 20, 2024. http://tinyurl.com/mududyr3
4. Kambhampati S, Saumoy M, Schneider Y, et al. Cost-effectiveness of polatuzumab vedotin combined with chemoimmunotherapy in untreated diffuse large B-cell lymphoma. Blood. 2022;140(25):2697-2708. doi:10.1182/blood.2022016624
5. Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386(4):351-363. doi:10.1056/NEJMoa2115304
