Post hoc Analysis of CLEAR Trial Shows Comparable OS Regardless of Subsequent Therapy in aRCC
Results from the phase 3 CLEAR trial showed lenvatinib plus everolimus to have a statistically significant improvement in progression-free survival and objective response rate compared with those given sunitinib.

Differences in subsequent treatments of anticancer medication affected the overall survival (OS) treatment effect in patients with advanced renal cell carcinoma (aRCC) treated with lenvatinib (Lenvima) plus everolimus (Afinitor) vs sunitinib (Sutent) in 2 of the 3 arms of the CLEAR trial (NCT02811861). Post hoc analyses adjusting for subsequent treatments found that OS was comparable between both arms, according to data presented at the 2022 International Kidney Cancer Symposium: Europe.1
Results from the multicenter, open-label, randomized, phase 3 study first showed that the lenvatinib plus everolimus arm had a statistically significant improvement in progression-free survival (PFS) and objective response rate (ORR) compared with the sunitinib arm. PFS was longer in the combination arm at a median of 14.7 months vs 9.2 months (HR, 0.65; 95% CI, 0.53-0.80; P < .0001) and ORR was higher at 53.5% vs 36.1%, respectively (odds ratio [OR], 2.15; 95% CI, 1.57%-2.93%).2
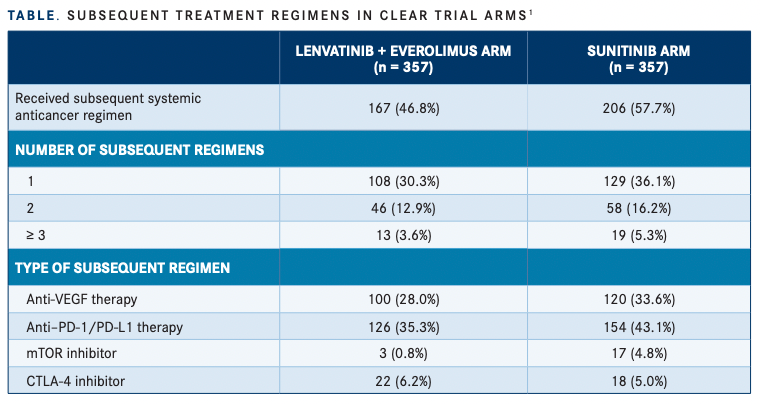
Median OS was not estimable (NE) in the CLEAR trial in either arm, but patients treated with sunitinib had a shorter median time from trial randomization to initiation of subsequent anticancer medication compared with patients in the lenvatinib plus everolimus arm, at 7.8 months vs 11 months, respectively.1 Moreover, investigators found an imbalance in the use of these medications, with 57.7% of patients in the sunitinib arm receiving any subsequent treatment compared with 46.8% in the combination arm. In the sunitinib arm, 129 patients (36.1%) had 1 subsequent regimen compared with 108 patients (30.3%) in the combination arm. Furthermore, 58 patients (16.2%) in the sunitinib arm had 2 subsequent regimens compared with 46 patients (12.9%) in the combination arm, and 19 patients (5.3%) vs 13 (3.6%), respectively, had 3 or more subsequent treatments (TABLE1 ).
Subsequent treatments included anti–PD-1/ PD-L1–containing therapies given to 43.1% of patients in the control arm vs 35.3% in the treatment arm; anti-VEGF therapy to 33.6% vs 28%, respectively; and mTOR inhibitors to 4.8% vs 0.8% of patients. However, more patients in the combination arm were given a CTLA-4 inhibitor at 6.2% vs 5.0%. Investigators then observed that in the US population subgroup, the number of patients who received subsequent anticancer medications was similar between the combination arm (62.9%) and control arm (65.6%).
The median OS with lenvatinib plus everolimus in this subgroup was NE (95% CI, 27.8 months-NE) and was also NE in the sunitinib arm (95% CI, 26 months-NE); however, the HR of 0.95 (95% CI, 0.51- 1.76) was comparable in this group. Therefore, investigators used a 2-stage estimation approach to adjust for the subsequent anticancer medications given in both arms of the study.
“The imbalance in the subsequent anticancer medication, including anti–PD-1/PD-L1–containing therapy, between the 2 arms (lenvatinib + everolimus vs sunitinib) may have impacted the OS treatment effect in CLEAR,” the study authors, led by Thomas E. Hutson, DO, PharmD, wrote in their poster.
When adjusting for any subsequent anticancer medication, the HR of the survival probability was 0.84 (95% CI, 0.64-1.12) and was 0.89 (95% CI, 0.68-1.19) when adjusting for next-line anti–PD-1/PD-L1 therapy. “When adjusted for the confounding effects of subsequent anticancer medication (via subgroup and statistical modeling analyses), OS was comparable for lenvatinib + everolimus versus sunitinib,” Hutson et al wrote.
In the CLEAR trial, patients were randomly assigned 1:1:1 to 3 different study arms consisting of lenvatinib plus pembrolizumab (Keytruda), lenvatinib plus everolimus, and sunitinib. Investigators then looked at the makeup of patient subgroups comparing just the lenvatinib plus everolimus arm (n = 357) and sunitinib arm (n = 357).
Patient characteristics were similar between both groups, with a median age of 62 years (range, 32-86) in the combination arm and 61 (range, 29-82) in the monotherapy arm. Slightly more patients in both groups came from Western Europe and North America vs rest of the world, at 56% vs 44% and 55.7% vs 44.3%, respectively. Furthermore, PD-L1 expression was similar as well, with 32.5% of patients in the lenvatinib plus everolimus arm having a combined positive score greater than 1 compared with 33.3% in the sunitinib arm.
Favorable prognostic risk per International Metastatic Renal Cell Carcinoma Database Consortium was reported in 27.5% of patients in the combination group vs 27.2% in the sunitinib group. Additionally, there were fewer patients with prior nephrectomy, at 72.8% in the combination arm compared with 77% in the control arm. Median duration of survival follow-up was similar at 27 months and 26 months in the lenvatinib and everolimus vs sunitinib arms, respectively, but the median duration of the first subsequent anticancer medication was longer, at 6.82 months (range, 0.03-30.72) vs 4.63 months (range, 0.03-34.4).
The safety profile assessed in the post hoc analyses was still consistent with the known safety profiles of the therapies. Treatment-emergent adverse events (TEAEs) that had fatal outcomes were observed, with the majority being attributed to progressive disease in both arms: 12.4% patients in the lenvatinib plus everolimus arm and 6.8% in the sunitinib arm. Treatment-related AEs that had a fatal outcome were observed in 3 patients in the combination arm and 1 in the monotherapy arm, and 7 patients in the combination arm had a fatal outcome not related to treatment vs 1 in the sunitinib arm.
“These data [show] that lenvatinib plus everolimus provides clinical benefit for patients with advanced RCC,” the investigators concluded.
REFERENCES
1. Hutson T, Choueiri T, Motzer R, et al. Post hoc analysis of the CLEAR study in advanced renal cell carcinoma: subsequent therapy and survival outcomes with lenvatinib + everolimus vs sunitinib. Poster presented at: 2022 International Kidney Cancer Symposium: Europe; April 22-24, 2022; Antwerp, Belgium. Abstract 18.
2. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/ NEJMoa2035716

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen