Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.

Although primarily a childhood disease, approximately 50% of acute lymphoblastic leukemia (ALL) cases occur in adults. Five-year overall survival (OS) among children younger than 15 years of age now exceeds 90%, and ranges from 70% to 80% in adolescents and young adults.1,2 However, adults 60 years of age and above, have 3-year survival rates of less than 20% and this decreases to less than 10% in adults above the age of 75.3
The major challenges for clinicians treating older patients with ALL surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies. Standard chemotherapy treatment approaches, such as hyper CVAD (cyclophosphamide, vincristine, doxorubicin [Adriamycin], and dexamethasone), are associated with induction mortality rates of 10%, with over one-third of patients dying while in remission, related to toxicity of treatment, and still 40% of patients relapse.4 Previous efforts to intensify chemotherapy for older adults to overcome resistance have resulted in high rates of treatment- related mortality.5,6
Recent efforts to de-intensify chemotherapy for older adults with ALL have resulted in improvements in treatment-related mortality, but unfortunately are also associated with significantly higher rates of relapse.7 Thus, current efforts and ongoing clinical trials are investigating how to decrease toxicity of treatment without sacrificing efficacy.
A number of newer class agents have recently been approved for treatment of relapsed and refractory B-cell ALL, including antibody-drug conjugates (ADCs), bispecific T-cell engagers (BiTEs), chimeric antigen receptor (CAR) T cells, and small molecule inhibitors.8 “
This suggests that for older patients, enrollment in a clinical trial is the best strategy,” Emily K. Curran, MD, assistant professor, University of Cincinnati College of Medicine in Ohio told Targeted Therapies in Oncology in an interview. “Ongoing clinical trials are exploring novel immunotherapies that are approved for relapsed and refractory disease in the upfront setting,” Curran continued.
Inotuzumab Ozogamicin (Besponsa)
For example, inotuzumab ozogamicin (InO), a CD22 ADC, is currently approved for the treatment of relapsed and refractory B-cell ALL based on the randomized phase 3 INO-VATE trial (NCT01564784).9 The trial data demonstrated an improvement in median OS to 7.7 months with inotuzumab ozogamicin compared with 6.2 months with standard of care, with 2-year OS rates of 22.8% and 10.0%, respectively (overall HR, 0.75; 97.5% CI, 0.57-0.99; P = .0105).9 Findings suggest that the burden of disease did not predict response. Hence, this is a good agent for upfront treatment/induction treatment.
Based on these encouraging results, ongoing trials are investigating whether using InO in the upfront setting can be used either in combination with chemotherapy or as a single agent for induction treatment for older patients with newly diagnosed Philadelphia (Ph) chromosome– negative B-cell ALL.
Jabbour E et al, evaluated the long-term outcomes of inotuzumab ozogamicin with or without blinatumomab (Blyncyto) and in combination with low-intensity chemotherapy.10 The open label phase 2 trial (NCT01371630) evaluated 80 patients (48 males) at a median age of 68 years. The primary end point was progression-free survival (PFS). After a median follow-up of 92.8 months, the 2-year PFS was 58.2% (95% CI, 46.7%-68.2%) and the 5-year PFS was 44.0% (95% CI, 31.2%54.3%), according to trial investigators.9
The protocol consisted of induction chemotherapy using the mini-hyper CVD (cyclophosphamide and dexamethasone at 50% dose reduction, methotrexate at 75% dose reduction, cytarabine at 83% dose reduction) regimen with inotuzumab ozogamicin (1.3-1.8 mg/m2). Maintenance therapy included dose-reduced POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone).
Researchers said that from patient 50 onwards, the study protocol was amended to fractionate inotuzumab ozogamicin to a maximum cumulative dose of 2.7 mg/m2 followed by blinatumomab for 4 cycles (cycles 5-8).10 At a median follow-up of 104.4 months (IQR, 6.6-89.2) before the regimen change, PFS was 34.7 months (95% CI, 15.0-68.3). At a median follow-up of 29.7 months (IQR, 8.841.0) after regimen change, the median PFS was 56.4 months (95% CI, 11.3-69.7).10
Results from the EWALL-INO phase 2 trial (NCT03249870) explored frontline inotuzumab ozogamicin combined with low-intensity chemotherapy in 131 older patients with CD22-positive Ph-negative ALL.11 Investigators noted that results confirmed that fractionated inotuzumab ozogamicin with low-intensity chemotherapy is active and well tolerated as a frontline therapy for older patients.
As a single-agent, inotuzumab ozogamicin induction followed by blinatumomab consolidation was shown to be highly active in newly diagnosed, Ph-chromosome– negative, B-cell ALL.12 Thirty-three patients were treated with inotuzumab ozogamicin (0.8 mg/m2 on day 1 and 0.5 mg/m2 on day 8).12 Patients who experienced cytoreduction moved on to induction IB if they had a complete remission or complete remission with incomplete count recovery. Patients who were not adequately cytoreduced were started on blinatumomab. After a median follow-up of 22 months, the 1-year event-free survival was 75% (95% CI, 61%-92%).12
In the open label, phase 2 INITIAL-1 trial (NCT03460522), 45 patients over 55 years of age received up to 3 cycles of inotuzumab ozogamicin and dexamethasone.13 All evaluable patients (n = 43) achieved a complete remission or complete remission with incomplete hematologic recovery.
After a median follow-up of 2.7 years, 1- and 3-year event-free survival was 88% (95% CI, 79%-98%) and 55% (95% CI, 40%-71%), respectively. Similarly, 1- and 3-year OS was 91% (95% CI, 82%-99%) and 73% (95% CI, 59%87%), respectively.
Blinatumomab
Blinatumomab is a bispecific T-cell engager directed toward CD3 and CD19. Blinatumomab is a fusion protein which consists of 2 single‐chain variable fragments arranged in tandem: the first binds the CD19 surface antigen of all B cells and the second targets the CD3 antigen of T cells.
Previously, findings from the phase 3 TOWER trial (NCT02013167) showed that blinatumomab led to longer OS than chemotherapy in adult patients with relapsed or refractory B-cell precursor ALL.14 A total of 405 patients were randomly assigned to receive either blinatumomab or standard-of-care chemotherapy.
OS was significantly longer in the blinatumomab group than in the chemotherapy group. The median OS was 7.7 months in the blinatumomab group and 4.0 months in the chemotherapy group (HR, 0.71; 95% CI, 0.55-0.93; P = .01).14
The agent’s effect on minimal residual disease was demonstrated in the BLAST trial (NCT01207388).15 In BLAST, among adult patients with minimal residual disease-positive ALL who were in hematologic remission after treatment with chemotherapy, 78% achieved a complete minimal residual disease response with blinatumomab.
In landmark analyses, complete minimal residual disease responders had longer relapse-free survival (RFS) (23.6 vs 5.7 months; P = .002) and OS (38.9 vs 12.5 months; P = .002) compared with MRD nonresponders.15 Blinatumomab has been shown to be well tolerated and effective in older patients with newly diagnosed Ph chromosome–negative B-cell ALL, according to findings from the SWOG 1318 trial (NCT02143414).16
A total of 29 patients were enrolled and received blinatumomab as induction for 1 to 2 cycles until response, which included complete response (CR) and CR with incomplete count recovery. Patients then received 3 cycles of consolidation with blinatumomab, followed by 18 months of POMP maintenance chemotherapy. At baseline, investigators reported that the median bone marrow blast count was 87% and patients were a median age of 75 years. Cytogenetic risk was poor in 10 patients (34%), and 5 of 14 patients (36%) evaluated had the Ph-like ALL gene signature. Nineteen patients (66%; 95% CI, 46%-82%) achieved CR. Three-year disease-free survival and OS estimates were 37% (95% CI, 17%-57%) and 37% (95% CI, 20%-55%), respectively. In patients with positive measurable residual disease, blinatumomab was shown to be effective, with a measurable residual disease conversion rate of 73%, a 3-year RFS rate of 63% (95% CI, 43%-77%), and a 3-year OS rate of 67% (95% CI, 46%-81%), according to a phase 2 study by Elias J Jabbour, MD, and colleagues (NCT02458014; TABLE).17
Blinatumomab was administered at a standard dosing of 28 micrograms daily as a continuous infusion for up to 5 cycles and up to 4 additional maintenance cycles. There were 37 patients enrolled at a median age of 43 years (range, 22-84 years). Twenty-seven patients (73%) were treated in first CR and 10 patients (27%) were treated in second CR and beyond. Eighteen patients (49%) had Ph chromosome–positive ALL and received concomitant tyrosine kinase inhibitor therapy. Twenty-three patients (62%) had a baseline MRD of 10-3 or greater. A median of 3 cycles (range, 1-9 cycles) were administered. Overall, 27 patients (73%) achieved MRD- negative remission.17
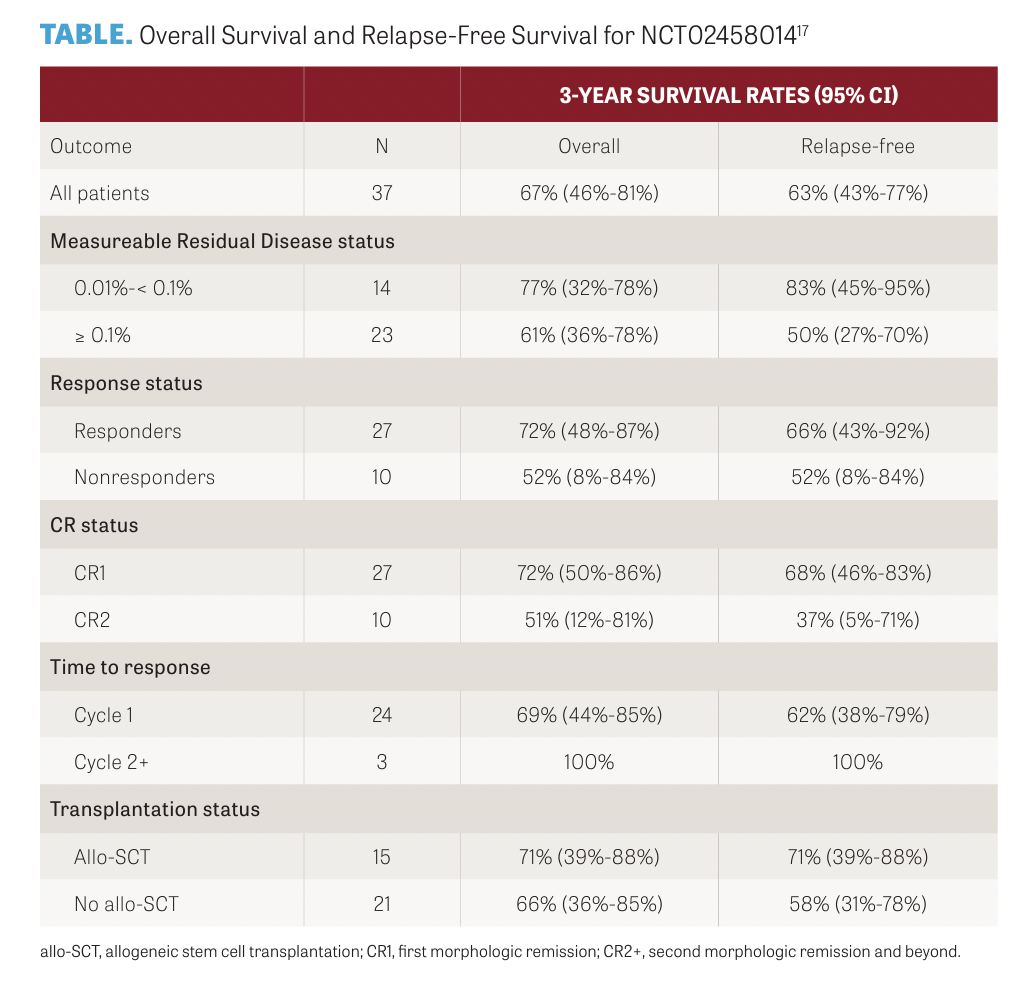
The 3-year RFS rates for patients with baseline MRD of 1 × 10−3 or higher and MRD of 1 × 10−3 or lower were 51% (95% CI, 27%-70%) and 83% (95% CI, 45%-95%), respectively. The rates of adverse events were consistent with previous studies of blinatumomab, reported the investigators.17
Curran said that results from the phase 3 ECOG-ACRIN E1910 trial (NCT02003222) showed that blinatumomab followed by consolidation chemotherapy achieved a 58% reduction in the risk of death compared with standard consolidation chemotherapy alone in adult patients with newly diagnosed MRD- negative B-cell ALL.18 Findings showed the median OS with blinatumomab plus chemotherapy was not reached compared with 71.4 months with chemotherapy alone (HR, 0.42; 95% CI, 0.24-0.75; P = .003). OS rates at a median 3.5 years were 83% and 65%, respectively.18
In the trial, patients underwent standard induction chemotherapy for about 2 months to induce a remission, followed by intensification chemotherapy for approximately 1 month. A total 224 patients were then randomized to receive intravenous blinatumomab for 2 monthly cycles, followed by 4 monthly cycles of consolidation chemotherapy and then 2 monthly cycles of blinatumomab (n = 112), or 4 monthly cycles of standard consolidation chemotherapy (n = 112).18 Patients, who were aged between 30 and 70 years and had newly diagnosed, BCR-ABL1–negative, B-lineage ALL, were also tested for MRD via 6-color flow cytometry at the time of randomization.18
“The trial showed that moving the bispecific agent, blinatumomab, into the upfront treatment, even in patients who are minimal residual disease negative, does have a benefit,” Curran said. “And so I think more and more, [immunotherapy agents] will probably be moved to the upfront setting.”
CAR T-Cell Therapy
Patients who were measurable residual disease positive with B-cell ALL were enrolled in a phase 1 open clinical trial (NCT03919526) to explore the safety and efficacy of CD19/CD22 bispecific targeted CAR-T cells.19 Fifteen out of 19 patients completed CAR T-cell infusion. All 15 patients experienced grade 3 or higher adverse events. A total of 4 patients (26.7%, 4/15) developed grade 1 or 2 cytokine-release syndrome (CRS) and all were in 5 × 106/kg CAR-T cell dose group. Two patients with grade 2 CRS were treated with tocilizumab and recovered quickly. The median time of CRS onset was 2 days (range, 1–8) after infusion, and the median duration was 1 day (range, 1–3).19
Ph Chromosome–Positive B-ALL
Another treatment strategy gaining momentum is moving immunotherapies to the upfront setting for Ph chromosome–positive B-ALL, Curran said. “A few years ago, there were promising data presented from the phase 2 D-ALBA trial (NCT02744768) in adults with Ph chromosome–positive ALL,” Curran said.20
Dasatinib plus glucocorticoids were administered, followed by 2 cycles of blinatumomab. The primary end point was a sustained molecular response in the bone marrow after this treatment.20
Of the 63 patients who were enrolled, a complete remission was observed in 98%. At the end of dasatinib induction therapy (day 85), 29% of the patients had a molecular response, and this percentage increased to 60% after 2 cycles of blinatumomab; the percentage of patients with a molecular response increased further after additional blinatumomab cycles.20 At a median follow-up of 18 months, OS was 95% and disease-free survival was 88%; disease-free survival was lower among patients who had an IKZF1 deletion plus additional genetic aberrations (CDKN2A or CDKN2B, PAX5, or both).20
“Many of us hope that by using this approach, we can maintain durable remissions and minimize the toxicity associated with chemotherapy,” Curran said.
REFERENCES:
1. Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33(27):2938-2948. doi:10.1200/JCO.2014.59.1636
2. Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–1559. doi:10.1182/blood-2018-10-881961
3. Geyer MB, Hsu Me, Devlin SM, Tallman MS, Douer D, Park JH. Overall survival among older US adults with ALL remains low despite modest improvement since 1980: SEER analysis. Blood.2017;129(13):1878–1881. doi:10.1182/ blood-2016-11-749507
4. O'Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113(8):2097-2101. doi:10.1002/cncr.23819
5. Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911-918. doi:10.1200/ JCO.2008.18.6916
6. Huguet F, Chevret S, Leguay T, et al. Intensified therapy of acute lymphoblastic leukemia in adults: report of the randomized GRAALL-2005 clinical trial. J Clin Oncol. 2018;36(24):2514-2523. doi:10.1200/JCO.2017.76.8192
7. Boissel N, Huguet F, Leguay T, et al. In adults with Ph-negative acute lymphoblastic leukemia (ALL), age-adapted chemotherapy intensity and MRD-driven transplant indication significantly reduces treatment-related mortality (TRM) and improves overall survival - results from the Graall-2014 trial. Blood. 2022;140(suppl 1):112–114. doi:10.1182/ blood-2022-157903
8. DuVall A, Sheade J, Anderson D, Yates SJ, Stock W. Updates in the management of relapsed and refractory acute lymphoblastic leukemia: an urgent plea for new treatments is being answered!. JCO Oncol Pract. 2022;18(7):479-487. doi:10.1200/OP.21.00843
9. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474-2487. doi:10.1002/cncr.32116
10. Jabbour E, Short NJ, Senapati J, et al. Mini-hyper-CVD plus inotuzumab ozogamicin, with or without blinatumomab, in the subgroup of older patients with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: long-term results of an open-label phase 2 trial. Lancet Haematol. 2023;10(6):e433-e444. doi:10.1016/S23523026(23)00073-X
11. Chevallier P, Leguay T, Kim R, et al. Fractionated inotuzumab ozogamicin combined with low-intensity chemotherapy in older patients with newly diagnosed CD22+ Philadelphia chromosome (Ph)-negative B-cell precursor (BCP) acute lymphoblastic leukemia (ALL): Results of the EWALL-INO study. Blood. 2022;140(suppl 1):6114–6116. doi:10.1182/ blood-2022-166035
12. Wieduwilt MJ, Yin J, Kour O, et al. Chemotherapy-free treatment with inotuzumab ozogamicin and blinatumomab for older adults with newly diagnosed, Ph-negative, CD22-positive, B-cell acute lymphoblastic leukemia: Alliance A041703. J Clin Oncol. 2023;41(suppl 16):7006. doi:10.1200/ JCO.2023.41.16_suppl.7006
13. Stelljes M, Raffel S, Alakel N, et al., Inotuzumab ozogamicin as induction therapy for patients older than 55 years with Philadelphia chromosome–negative B-precursor ALL. J Clin Oncol. 2023;23:00546. doi:10.1200/JCO.23.00546
14. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi:10.1056/ NEJMoa1609783
15. Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531. doi:10.1182/blood-2017-08-798322
16. Advani AS, Moseley A, O'Dwyer KM, et al. SWOG 1318: A Phase II trial of blinatumomab followed by POMP maintenance in older patients with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia. J Clin Oncol. 2022;40(14):1574-1582. doi:10.1200/JCO.21.01766
17. Jabbour EJ, Short NJ, Jain N, et al. Blinatumomab is associated with favorable outcomes in patients with B-cell lineage acute lymphoblastic leukemia and positive measurable residual disease at a threshold of 10-4 and higher. Am J Hematol. 2022;97(9):1135-1141. doi:10.1002/ajh.26634
18. Litzow MR, Sun Z, Paletta E, et al. Consolidation therapy with blinatumomab improves overall survival in newly diagnosed adult patients with B-Lineage acute lymphoblastic leukemia in measurable residual disease negative remission: results from the ECOG-ACRIN E1910 randomized Phase III National Cooperative Clinical Trials Network Trial. Blood. 2022;140(suppl 2):LBA-1. doi:10.1182/blood-2022-171751
19. Niu J, Qiu H, Xiang F, et al. CD19/CD22 bispecific CAR-T cells for MRD-positive adult B cell acute lymphoblastic leukemia: a phase I clinical study. Blood Cancer J. 2023;13(1):44. doi:10.1038/s41408-023-00813-x
20. Foà R, Bassan R, Vitale A, et al. Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383(17):1613-1623. doi:10.1056/NEJMoa2016272
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More