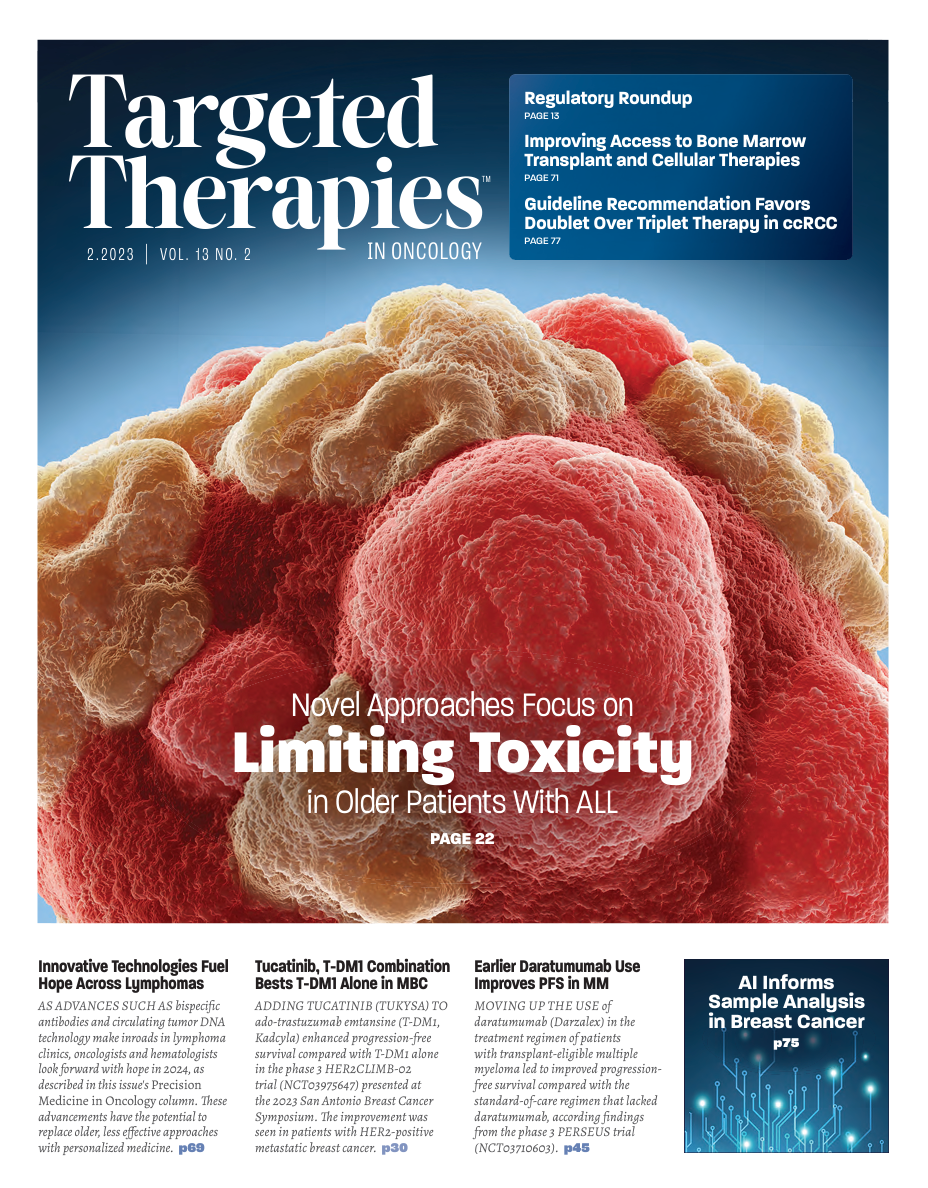
Improving Access to Bone Marrow Transplant and Cellular Therapies
Stem cell treatments offer hope for a variety of conditions. However, access to these potentially life-altering procedures is often hampered by social factors such as socioeconomic status, race, cultural background, and geographic location.
Hematopoietic stem cell transplantation (HCT) and cellular therapies offer life-saving potential for individuals facing hematologic malignancies, bone marrow failure syndromes, some autoimmune disorders, and certain solid tumors. However, various social vulnerabilities significantly influence access to these critical procedures, including socioeconomic status, race, culture, and geography.1-8 Other vital factors that affect access include insurance coverage, associated costs (co-payments, travel, housing, and time away from work), the knowledge of referring oncologists, donor availability, caregiver support, patient comorbidities, health literacy, and personal beliefs. There is an expanding interest in the HCT and cellular therapy community in abrogating these potential barriers to transplant.
Notably, recent strides in access to allogeneic (allo) HCT have focused on refining alternative donor transplantation methods, including haploidentical and mismatched unrelated transplants. The integration of posttransplant cyclophosphamide has yielded outcomes comparable to those of matched unrelated donor transplants.9 This is extremely important due to the uneven distribution of potential donors in the National Marrow Donor Program (NMDP) registry, with significant discrepancies based on ethnicity. Evidence from one study revealed a 75% likelihood of finding a full (8/8) human leukocyte antigen (HLA) match in the NMDP registry for patients of White European descent, contrasting with only 16% for Black people of South or Central American ancestry.10 With the use of alternative donors, over 95% of patients have at least 1 HLA-haploidentical first-degree donor.11
An additional obstacle to alloHCT is tied to the location of transplant centers, especially given the prolonged stay of patients and their caregivers during the transplant process. Despite the presence of 229 transplant facilities in the United States (185 for adults and 111 for pediatric care), the concentration of these centers, particularly on the East Coast, poses a signifi cant challenge for patients residing in more rural areas.7 Although 66% of the population lives within 60 minutes of travel time and 94% within 3 hours, the burden of frequent posttransplant visits, coupled with unaddressed housing and caregiver expenses not covered by insurance, creates substantial financial barriers for many.
Some patients may have potential donors and easy access to a transplant center, but another critical issue is the lack of referral for a transplant evaluation. Some existing databases used to analyze disparities, whether institutional or national (such as the Center for International Blood and Marrow Transplant Research), focus primarily on those who have undergone HCT, neglecting individuals with conditions who could benefit from transplantation but who have not been evaluated.1 A recent prospective study by Scott et al assessed transplantation referral patterns among 778 patients with high-risk myelodysplastic syndromes or acute monocytic leukemia (AML) in the Connect Myeloid Disease Registry undergoing treatment at community versus academic sites.12 Data from this study showed that more patients at academic sites were considered potentially eligible for transplant compared with the community sites (43.9% vs 27.9%; P < .0001), with multivariate analysis demonstrating ineligibility was based on age or comorbidities. In this study, 20% of patients were not assessed at all for transplant eligibility.
The aging population has become a particular focus in the transplant community. Over 40% of patients receiving allogeneic transplants in the United States are 60 years or older based on Center for International Blood and Marrow Transplant Research (CIBMTR) data.13,14 Artz et al examined the preferred post remission consolidation therapy (alloHCT vs consolidative chemotherapy) for older adults with AML in first complete remission (CR1). Results were worse initially in the alloHCT group, with an improvement over time. The overall survival rate of those who received an HCT was inferior during the first 9 months after transplant compared with those who did not receive HCT (HR, 1.52; P = .02). Conversely, after year 5, those who received an alloHCT had improved overall survival (28.6% vs 13.8%; HR, 0.53; P < .0001).14,15 These data highlight the importance of a transplant referral for patients of all ages with potentially eligible diagnoses.
Recent efforts have also aimed at expanding access to patients with active disease or measurable residual disease.
These efforts include clinical trials with novel targeted conditioning approaches, manipulated cellular therapy products, and targeted radiation approaches.
Favorable outcomes are achievable, and referral to a transplant center should be considered.
Barriers to accessing bone marrow transplants and cellular therapies are complex and multifaceted. Addressing them requires collective efforts to ensure equitable access and improved outcomes for all eligible patients, considering social, economic, and geographic factors.
Amanda Blackmon, DO, is assistant clinical professor, Division of Leukemia, Department of Hematology & Hematopoietic Cell Transplantation, City of Hope Comprehensive Cancer Center, Duarte, California. Idoroenyi Amanam, MD, is Assistant Professor, Division of Leukemia, Department of Hematology & Hematopoietic Cell Transplantation, City of Hope Comprehensive Cancer Center, Duarte, California.