New Approaches to the Treatment of Relapsed or Refractory Diffuse Large B-cell Lymphoma
In the United States, the most common of the aggressive non-Hodgkin lymphomas (NHLs) is diffuse large B-cell lymphoma (DLBCL), which accounts for between 22% and 24% of newly diagnosed B-cell NHL cases.1 Although DLBCL can affect children and young adults, it is most commonly diagnosed in individuals between the ages of 65 and 74 years, with a median age at diagnosis of 66 years.2,3 Given the aggressive nature of DLBCL, patients often present with lymphadenopathy, extranodal involvement, and other constitutional symptoms that
require immediate treatment.1
The treatment spectrum for DLBCL has expanded significantly in recent years, particularly for patients with relapsed or refractory (R/R) disease. Mechanisms of action differ greatly among agents, reflecting the complex pathophysiology and genetic variations of the disease. This article reviews the advances in DLBCL understanding that have led to the approval of new agents and
subsequent utilization of new mechanisms.
Standards of Care and Unmet Needs in Relapsed or Refractory Disease
The current standards of care for first-line DLBCL treatment include the combination chemoimmunotherapy regimen of rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone (R-CHOP). The varying numbers of cycles and use in combination with or without radiotherapy (RT) depends upon the stage of disease at presentation.1 The addition of rituximab to CHOP was associated with a 2-year event-free survival of 57% in elderly patients in a 2002 randomized trial (LNH-98.5), which, along with results of other trials, led to the FDA approval of this combination therapy.4,5 Although durable remission can be achieved with R-CHOP in about 60% of patients, its use has resulted in poorer long-term outcomes for patients with double-hit and
triple-hit lymphomas (DHL and THL).1
In 2007, the International Harmonization Project issued guidelines on malignant lymphoma response criteria, defining relapsed disease as consisting of new lesions greater than 1.5 cm in any axis during or after the completion of therapy or a 50% or greater increase in the sum of the product of diameters of a previously involved node(s) or other lesion(s).6 The authors also defined refractory, or progressive, disease as entailing a 50% or greater increase in the size of a lymph node with a prior short-axis diameter of less than 1.0 cm to a size of 1.5 cm × 1.5 cm (or a long-axis size of > 1.5 cm).6
For patients with R/R disease, high-dose chemotherapy and autologous stem cell transplant (ASCT) may offer the chance for cure, but several factors may limit the utility of this approach. For example, in the treatment of patients with MYC-positive R/R DLBCL, ASCT is considered controversial because it has produced poorer outcomes in patients with DHL.1 Additionally, patients who are older or have comorbidities may be inappropriate candidates for this approach,7 and patients with disease that is unresponsive to second-line chemotherapy may have poorer prognoses (ie, poorer rates of long-term survival) and incur added toxicity from the chemotherapy.7 Even when including patients who undergo high-dose, salvage chemotherapy and subsequent ASCT, patients with R/R DLBCL have a 1-year survival rate of 28%.1 Hence, in a search for improved outcomes in the R/R setting, clinical studies have focused on DLBCL subtypes, especially in those ineligible for transplant or who have relapsed following transplant.1
Another option for patients in the relapsed setting is chimeric antigen receptor (CAR) T-cell therapy, which entails the genetic modification of autologous T cells via cloned DNA plasmids carrying a viral recombinant vector in addition to T-cell receptor-expressing genes. CAR T-cell therapy plays an important role in the R/R DLBCL setting, with reported 2-year remissions and a complete response (CR) rate in 40% of patients and 25% DHL/THL patients.1 Other therapeutic classes that have been explored for DLBCL include phosphoinositide 3-kinase (PI3K) inhibitors, B-cell lymphoma 2 (BCL2) inhibitors, and checkpoint inhibitors.1,8-10
New Mechanisms in Relapsed or Refractory DLBCL Treatment
Given reduced survival in patients who are unresponsive to subsequent lines of therapy and the toxicity involved, a great need exists for novel agents in the R/R DLBCL setting. Recent entrants to the R/R DLBCL treatment landscape include the antibody-drug conjugate (ADC) polatuzumab vedotin-piiq, the selective inhibitor of nuclear export, selinexor, and the monoclonal antibody tafasitamab-cxix (TABLE 111-20).
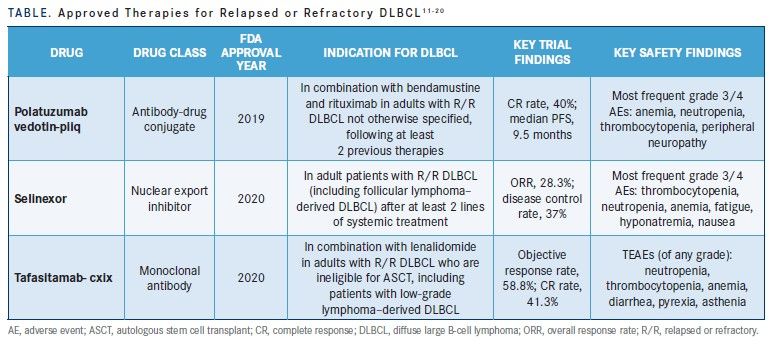
Polatuzumab vedotin-piiq
Polatuzumab vedotin-piiq was approved by the FDA in 2019 and is indicated in combination with bendamustine and rituximab in adults with RR DLBCL not otherwise specified, following at least 2 previous therapies.11 It is an ADC wherein the monoclonal antibody is linked to an antimitotic agent, monomethyl auristatin E (MMAE). The ADC targets the B-cell surface protein CD79B and, after binding to the surface protein, is internalized by the cell. Lysosomal enzymes then cleave the link between the antibody and MMAE, the latter of which binds microtubules, thereby inhibiting cell division and inducing apoptosis.11
A 2020 phase 1b/2 study (NCT02257567) randomized patients with R/R DLBCL who were ineligible for ASCT to receive polatuzumab vedotin-piiq with bendamustine and rituximab (pola-BR) or bendamustine and rituximab (BR) alone.12 The phase 2 primary end point was CR; secondary end points included overall response rate (ORR) at end of treatment, superior overall response, duration of response (DOR), and progression-free survival (PFS) assessed per independent review committee (IRC).12 With a median follow-up of 22.3 months, the CR was significantly higher in the pola-BR group (40% vs 17.5% in the BR group; P = .026).12 Overall survival rate was also significantly higher in the pola-BR group (12.4 vs 4.7 months in the BR group; HR, 0.42; 95% CI, 0.24-0.75; P = .002).12 Similarly, median PFS was significantly longer at 9.5 months in the pola-BR group compared with 3.7 months in the BR group (HR, 0.36; 95% CI, 0.21-0.63; P < .001).12 Also, DOR was longer at 12.6 months in the pola-BR group vs 7.7 months in the BR group (HR, 0.47; 95% CI, 0.19-1.14).12 Finally, the pola-BR group had a 58% reduction in risk of death compared with the BR group (HR, 0.42; 95% CI, 0.24-0.75; P = .002).12 In terms of safety, grade 3/4 anemia, neutropenia, thrombocytopenia,
and peripheral neuropathy occurred more frequently in the pola-BR group than in the BR group.12 Polatuzumab vedotin-piiq was deemed an effective agent that might provide a therapeutic option for patients with R/R DLBCL who were not ideal candidates for CAR T-cell therapy.12
Selinexor
In 2020, selinexor was approved by the FDA for use in adult patients with R/R DLBCL (including follicular lymphoma-derived DLBCL) after at least 2 lines of systemic treatment.13 Selinexor inhibits nuclear export of tumor suppressor proteins by blocking exportin 1.13
The FDA approval was based on results of the open-label single-arm phase 2 SADAL trial (NCT02227251), which included patients 18 years or older with DLBCL (based on pathologic confirmation) with an Eastern Cooperative Oncology Group (ECOG) score of 2 or less, who had 2 to 5 lines of prior therapy, and who had progressed following or were ineligible for ASCT.14 The primary end point of the SADAL trial was ORR (comprising patients with CR or PR per 2014 Lugano criteria), with secondary end points consisting of DOR and disease control rate.14 Patients received the 60-mg oral selinexor on the first and third day of each week until disease progression or unacceptable toxicity occurred.14
The updated phase 2b ORR was 28.3% with a disease control rate of 37% (95% CI, 28.6-46.0). Of 36 responders, CRs were reported in 13 evaluable patients and PRs were reported in 23 patients. At a median follow-up of 11.1 months, the median DOR was 9.3 months (95% CI, 4.8-23.0). For those with a CR, median DOR was 23.0 months (95% CI, 10.4-23.0); median DOR was 4.4 months for those with a PR (95% CI, 2.0–not evaluable).14,15 To address potential differences by subtype, the SADAL trial also included a subgroup analysis of patients with the germinal center B-cell (GCB)–like subtype (n = 59), which demonstrated an ORR of 33.9%, a 14% CR rate, and a 20% PR rate, whereas the patients with a non-GCB subtype (n = 63) had an ORR of 20.6%. At the time of data cutoff, 7%
(n = 9) of patients showed continuing response.14,15 The SADAL trial also included 5 patients with the unclassified subtype, in 1 of whom a CR was achieved and in 2 of whom a PR was achieved.15
With respect to safety, 98% of patients in the SADAL trial had at least 1 treatment-emergent adverse event (TEAE). The most frequent grade 3/4 events were thrombocytopenia, neutropenia, anemia, fatigue, hyponatremia, and nausea.14 Among serious AEs affecting 48% of patients, the most common were pyrexia, pneumonia, and sepsis.14 Gastrointestinal AEs were
reported in 80% of patients, hyponatremia in 61%, and central neurologic events (which included dizziness and altered mental status) in 25%.16 Trial investigators concluded that selinexor improved survival considerably and that it presented a nonchemotherapy oral option for patients with R/R DLBCL.14
Tafasitamab-cxix
Tafasitamab-cxix is a CD19-targeting monoclonal antibody that gained FDA approval in 2020 for use with lenalidomide in adults with R/R DLBCL who are ineligible for ASCT, including patients with low-grade lymphoma derived DLBCL.17 Tafasitamab-cxix binds to the pre-B and mature B-lymphocyte surface antigen CD19, which is expressed in DLBCL and other B-cell malignancies.17 Tafasitamab-cxix, once bound to CD19, facilitates B-lymphocyte lysis via apoptosis and immune effector mechanisms that encompass antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis.17
The FDA approval of tafasitamab-cxix was based on data from the phase 2, single-arm, multicenter, open-label L-MIND trial (NCT02399085).17,18 The L-MIND trial included patients 18 years or older with R/R DLBCL who had received 1 to 3 previous therapies (≥ 1 of which incorporated a CD20-directed regimen), had an ECOG score of 0 to 2, and were ASCT ineligible.18 Patients were administered tafasitamab-cxix and lenalidomide in 28-day cycles and continued to receive tafasitamab-cxix every 2 weeks after cycle 12 until disease progression.18 Objective response rate (ie, PR and CR) was the primary end point per IRC, which implemented PET imaging; secondary end points included investigator-assessed objective response rate, DOR, OS, PFS, biomarker analyses, and safety.18 Eighty patients were included in the full analysis set (FAS), receiving tafasitamab-cxix plus lenalidomide.18 Of the FAS, the objective response rate was 60.0% (95% CI, 48.4%-70.8%) and the CR rate was 42.5% (34/80).18 The rate of patients achieving a 12-month DOR rate was comparable across subgroups, with 70.5% of patients who received 1 prior line of therapy achieving a 12-month DOR (95% CI, 47.2%-85.0%) and 72.7% of patients who had 2 or more prior lines of therapy achieving a 12-month DOR (95% CI, 46.3%-87.6%).18
Outcomes in patients with GCB DLBCL (n = 37) were promising, with an objective response rate of 48.6%, a 12-month DOR rate of 53.5%, and a 12-month OS rate of 65.4% (based upon the Hans algorithm). Outcomes in patients with non-GCB DLBCL (n = 21) were an improvement over those with the GCB subtype, with an objective response rate of 71.4%, a 12-month DOR rate of 83.1%, and a 12-month OS rate of 84.2%.18 IRC-evaluated data from a 2-year follow up of the L-MIND trial showed an objective response rate of 58.8% (47/80) and CR rate of 41.3% (33/80).19 The 2-year follow up data also showed a median DOR of 34.6 months, with a 31.6-month median OS and a 16.2-month median PFS.19
Safety data from the preliminary L-MIND trial results showed that the most frequent TEAEs (of any grade) were neutropenia (48%), thrombocytopenia (32%), anemia (31%), diarrhea (30%), pyrexia (22%), and asthenia (20%).20 A lenalidomide dose reduction was required in 42% of patients; 72% of patients could remain on daily lenalidomide at 20 mg or higher.20 Trial investigators concluded that the combination of tafasitamab-cxix and lenalidomide was well tolerated and did not lead to compounded AEs.20
Conclusions and Future Directions
The promising data from recent trials—particularly from their DLBCL subtype based subgroups—underscore the importance of understanding the unique prognoses and responses that these subtypes confer on patient outcomes. The establishment of DLBCL subtypes as prognostic and therapeutic response factors has fueled a search for more specific molecular targets in the disease process. In addition, the importance of subtype characterization is evidenced by ongoing diagnostic assay development (for use in conjunction with immunohistochemistry). As exemplified by the patient populations in these trials, new therapeutic options with distinct mechanisms of actions are needed for patients with R/R DLBCL who are ineligible for ASCT. Multiple studies of targeted agents in the R/R DLBCL setting are under way that include CAR T-cell, bispecific T-cell engager, programmed death receptor 1 (PD-1) inhibitor, and BCL2 inhibitor therapies.1 Continued development of clinically applicable diagnostics holds promise for improved prognostic capability and assessment of therapeutic response. With improved diagnostics, further elucidation of DLBCL-driver mutations can continue to provide additional DLBCL subtype-specific options and, hence, more treatments tailored to individual patients.
References
1. Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604-616. doi:10.1002/ajh.25460
2. Diffuse large B-cell lymphoma. Lymphoma Research Foundation. Accessed October 12, 2020. https://lymphoma.org/aboutlymphoma/nhl/dlbcl/
3. Cancer stat facts: NHL – diffuse large B-cell lymphoma (DLBCL). National Cancer Institute. Accessed October 12, 2020. https://seer.cancer.gov/statfacts/html/dlbcl.html
4. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. doi:10.1056/NEJMoa011795
5. Rituxan plus CHOP approved for diffuse large B-cell lymphoma. Cancer Network. February 28, 2006. Accessed November 6, 2020. https://www.cancernetwork.com/view/rituxan-plus-chop-approved-diffuse-large-b-cell-lymphoma
6. Cheson BD, Pfistner B, Juweid ME, et al; International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. doi:10.1200/JCO.2006.09.2403
7. Elstrom RL, Martin P, Ostrow K, et al. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk. 2010;10(3):192-196. doi:10.3816/CLML.2010.n.030
8. Oki Y, Kelly KR, Flinn I, et al. CUDC-907 in relapsed/refractory diffuse large B-cell lymphoma, including patients with MYC-alterations: results from an expanded phase I trial. Haematologica. 2017;102(11):1923-1930. doi:10.3324/haematol.2017.172882
9. Ansell S, Gutierrez ME, Shipp MA, et al. A phase 1 study of nivolumab in combination with ipilimumab for relapsed or refractory hematologic malignancies (CheckMate 039). Blood.
2016; 128(22):183. doi:10.1182/blood.V128.22.183.183
10. Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698-2704. doi:10.1200/JCO.2015.65.9789
11. POLIVY. Prescribing information. Genentech, Inc; 2020. Accessed October 22, 2020. https://www.gene.com/download/pdf/polivy_prescribing.pdf
12. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172
13. XPOVIO. Prescribing information. Karyopharm Therapeutics, Inc; 2020. Accessed October 22, 2020. https://www.karyopharm.com/wp-content/uploads/2019/07/NDA-212306-SN-0071-Prescribing-Information-01July2019.pdf
14. Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7(7):e511-e522. doi:10.1016/S2352-3026(20)30120-4
15. Karyopharm reports updated data from the phase 2b SADAL study at the 2019 International Conference on Malignant Lymphoma. News release. Karyopharm. June 19, 2019.
Accessed June 28, 2020. https://www.globenewswire.com/news-release/2019/ 06/19/
1871363/0/en/Karyopharm-Reports-Updated-Data-from-the-Phase-2b-SADAL-Study-at-the-2019-International-Conference-on-Malignant-Lymphoma.html
16. FDA approves selinexor for relapsed/refractory diffuse large B-cell lymphoma. News release. FDA. June 22, 2020. Accessed June 28, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selinexor-relapsedrefractory-diffuse-large-b-cell-lymphoma
17. Monjuvi. Prescribing information. MorphoSys US Inc; 2020. Accessed October 22, 2020. https://www.monjuvi.com/pi/monjuvi-pi.pdf
18. Duell J, Maddocks KJ, Gonzalez-Barca E, et al. Subgroup analyses from L-Mind, a phase II study of tafasitamab (MOR208) combined with lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Blood. 2019;134(suppl 1):1582. doi:10.1182/blood-2019-122573
19. MorphoSys and Incyte announce long-term follow-up results from L-MIND study of tafasitamab in patients with r/r DLBCL. News release. Morpho-Sys. May 14, 2020. Accessed June 26, 2020. https://www.morphosys.com/media-investors/media-center/morphosys-and-incyte-announce-long-term-follow-up-results-from-l-mind
20. Salles GA, Duell J, González-Barca E, et al. Single-arm phase II study of MOR208 combined with lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma: L-Mind. Blood. 2018;132(suppl 1):227. doi:10.1182/blood-2018-99-113399
Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen
