More Approaches Are Emerging in TP53-Mutated AML
Although the need for novel approaches to treat patients with TP53-mutated acute myeloid leukemia remains urgent, cautious optimism may be in order given recent data from clinical trials and upcoming studies.

Prognosis for patients with tumor protein p53 (TP53)-mutated acute myeloid leukemia (AML) is poor; approximately 12% of patients survive for 2 years, even when receiving intensive treatment.1 Likewise, results of an analysis presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition found that median survival in this setting was only 6 to 7 months with the 3 most commonly used standards of care.2 Although the need for novel approaches to treat patients with TP53-mutated AML remains urgent, cautious optimism may be in order given recent data from clinical trials and upcoming studies.
TP53 in AML
The tumor suppressor gene TP53, located on chromosome 17p13, encodes the transcription factor p53, which carries out multiple antiprolif-erative functions in AML and many other cancer types.3 Mutations in TP53 are present in approx-imately 10% of patients with AML, who may also have other mutations such as FLT3, KRAS, NRAS, KIT, PTPN11, and NF1, contributing to a complex karyotype when 3 or more such chromosomal abnormalities are present.4 Conventional treatment for patients with AML consists of cytarabine and an anthracycline, which achieves an 80% complete response rate for patients with TP53 wild-type AML, but only 20% to 40% for patients with TP53-mutated AML.5
Magrolimab
One of the agents furthest along in development for TP53-mutated AML is magrolimab, which is being evaluated in the phase 3 ENHANCE-2 trial (NCT04778397) for patients with previously untreated TP53-mutated AML. Magrolimab is a monoclonal antibody that targets CD47, which is upregulated on AML cells and helps them evade the innate immune system.6 Magrolimab prevents the SIRP-α receptor on macrophages from recognizing CD47, allowing macrophages to phagocytose cancer cells.6 According to Nadal G. Daver, MD, ENHANCE-2 is the first study of AML that is TP53-mutated specific. Patients are randomly assigned to receive azacitidine (Vidaza) plus magrolimab versus physician’s choice of venetoclax plus azacitidine or 7+3 chemotherapy.
“If this trial is positive, magrolimab would be the first TP53-specific mutationally-directed approval that we have seen in myeloid malignancies,” Daver said in an interview with Targeted Therapies in Oncology™. “There’s a lot of enthusiasm based on previous results both in myelodysplastic syndrome (MDS) and AML.” Daver is an associate professor in the Department of Leukemia at The University of Texas MD Anderson Cancer Center in Houston.
Eytan M. Stein, MD, chief of the Leukemia Service at Memorial Sloan Kettering Cancer Center in New York, New York, pointed out that it will be important to wait for the results of randomized phase 3 studies before drawing conclusions. However, “magrolimab has shown efficacy in TP53-mutated AML when combined with azacitidine, with response rates exceeding those expected with azacitidine alone,” Stein said during an interview with Targeted Therapies in Oncology™. The estimated primary completion data for the ENHANCE-2 trial is August 2024.
Eprenetapopt
Eprenetapopt (APR-246) is a small molecule p53 reactivator that has been tested in both solid tumors and hematologic malignancies but demonstrated a negative result in a phase 3 front-line trial of TP53-mutated high-risk MDS.7 Although the results showed a higher rate of complete responses of 33.3% (95% CI, 23.1%-44.9%) compared with 22.4% (95% CI, 13.6%-33.4%) in the azacitidine monotherapy arm, (P = .13), the difference between the 2 arms did not meet the predefined threshold for statistical signifi cance.7 The manufacturer recently stated that it has no ongoing clinical trials for eprenetapopt.8
TIM-3 Inhibitors
Also under investigation are TIM-3 inhibitors. TIM-3 is an immune checkpoint that is overexpressed on leukemic stem cells and absent on normal hematopoietic stem cells.9 The anti–TIM-3 monoclonal antibody sabatolimab has been evaluated in combination with a hypomethylating agent in a phase 1b study (NCT03066648) of patients with high or very high-risk MDS (n = 53) or newly diagnosed AML.10 The regimen appeared to be well tolerated, with hematologic and infectious toxicities being the most common grade 3 or higher adverse events. A triplet regimen composed of azacitidine, venetoclax (Venclexta), and sabatolimab is being evaluated in the phase 2 STIMULUS-AML1 study (NCT04150029) with an expected primary completion date of March 2026.
MDM2 Inhibitors
Several MDM2 inhibitors are in development and may hold promise for AML. MDM2 is a key negative regulator of TP53 and its expression. MDM2 inhibition is aimed at reactivating wild-type p53 and its tumor suppressor functions. MDM2 inhibitor idasanutlin (RG7388) has been evaluated in a phase 1b trial (NCT02670044) in combination with venetoclax in patients with relapsed/refractory AML who are not eligible for chemotherapy. The findings demonstrated efficacy and tolerability, warranting further investigation.11 However, the phase 3 MIRROS trial (NCT02545283), which added idasanutlin to cytarabine, did not improve overall survival in patients with relapsed/refractory AML.12 Other agents in this class being investigated in early-stage clinical trials include AMG 232 (navtemadlin, KRT-232), milademetan,13 and siremadlin (HDM201).14
Select Clinical Trials Recruiting for TP53-Mutated AML
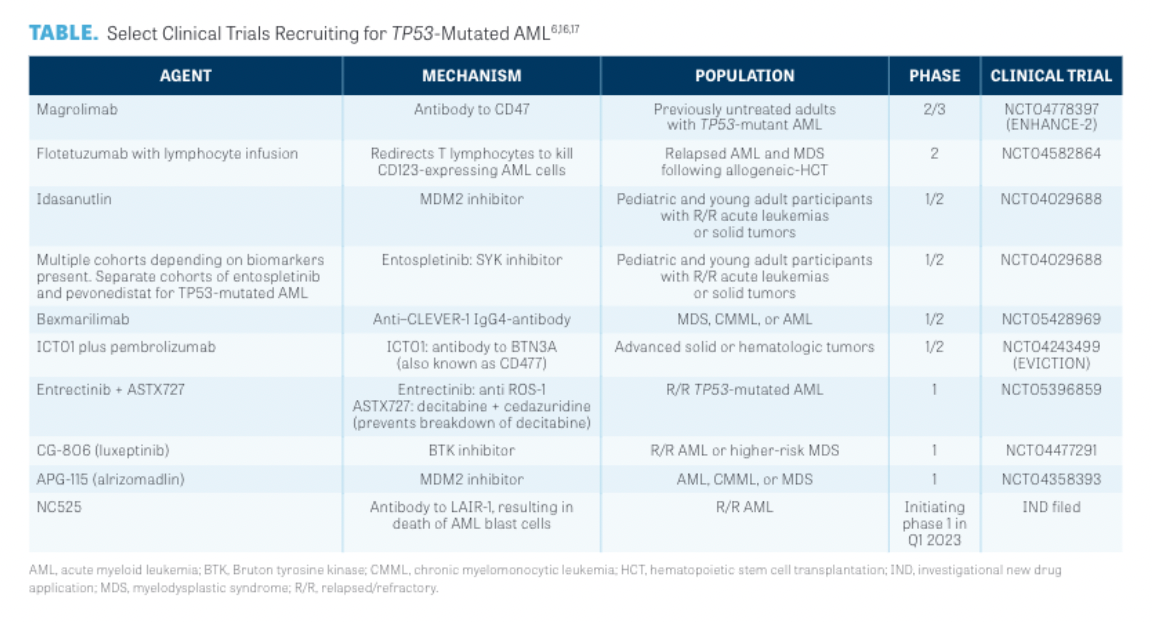
Other Immune-Targeted Approaches
“Despite the poor outcomes in this setting, several novel immune and cellular therapy approaches, including those with potentially p53-agnostic mechanisms of action, could hold promise,” said Abhishek Maiti, MD, during an interview with Targeted Therapies in Oncology™. Maiti is an assistant professor in the Department of Leukemia at The University of Texas MD Anderson Cancer Center. Multiple additional immune approaches are in early-stage clinical trials, including bispecific antibodies.15 For example, flotetuzumab (MGD006) is a bispecific antibody that targets CD3ε and CD123, redirecting polyclonal effectors to kill tumor cells. This agent has demonstrated efficacy and tolerability in a clinical trial of patients with AML relapsing on induction therapy16and is in an ongoing clinical phase 1 study (NCT04681105). Other promising immunotherapeutic approaches, according to Maiti, include bexmarilimab, an IgG4-an-tibody that stimulates tumor-associated macrophages; ICT01, an antibody to BTN3A (also known as CD277) that activates the innate immune system; and NC525, an antibody to LAIR-1, which results in death of AML blast cells. The TABLE highlights ongoing research.6,16,17
Future Research
Study findings in TP53-mutated AML highlight the critical need to enter patients as soon as possible into clinical trials, Daver said during the interview. “It’s our recommendation to community oncologists to send patients to clinical trials when they’re newly diagnosed,” Maiti said.
Rory Shallis, MD, an assistant professor of hematology in the Department of Internal Medicine at Yale School of Medicine in New Haven, Connecticut, noted that “there are literally thousands of mutations that can affect TP53 gene function, and some appear to be insufficient to be associated with the terrible disease biology that we recurrently observe.” According to Shallis, full profiling of TP53 alterations in large databases and larger and more inclusive clinical trials are needed to fully validate the applicability to many disease subgroups. "This is important as there is much variance within this specific molecular subgroup,” he said, adding that it is his hope that these efforts will lead to more therapeutic options. “All we need is 1 or 2 agents to really be the game changer that we need to start definitively impacting outcomes,” Shallis said.
REFERENCES
1. Grob T, Al Hinai ASA, Sanders MA, et al. Molecular characterization of mutant TP53 acute myeloid leuke-mia and high-risk myelodysplastic syndrome. Blood. 2022;139(15):2347-2354. doi:10.1182/blood.2021014472
2. Daver N, Iqbal S, Huang J, et al. Clinical characteristics and overall survival among acute myeloid leukemia (AML) patients with TP53 gene mutation (TP53m) or chromosome 17p deletion (17p del). Presented at 64th American Society of Hematology Annual Meeting and Exposition; December 10-13, 2022; New Orleans, LA. Abstract 603.
3. Vogelstein B, Lane D, Levine AJ. Surfi ng the p53 network. Nature. 2000;408(6810):307-310. doi:10.1038/35042675
4. DiNardo CD, Cortes JE. Mutations in AML: prognostic and therapeutic implications. Hematology Am Soc Hematol Educ Program. 2016;2016(1):348-355. doi:10.1182/asheduca-tion-2016.1.348
5. Shallis RM, Bewersdorf JP, Stahl MF, Halene S, Zeidan AM. Are we moving the needle for patients with TP53-mutated acute myeloid leukemia? Cancers (Basel). 2022;14(10):2434. doi:10.3390/cancers14102434
6. Jaiswal S, Jamieson CH, Pang WW, et al. CD47 is upregu-lated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271-85. doi:10.1016/j.cell.2009.05.046
7. Aprea Therapeutics announces results of primary endpoint from phase 3 trial of eprenetapopt in TP53 mutant myelodysplastic syndromes (MDS). News release. Aprea Therapeutics. December 29, 2020. Accessed November 29, 2022. https://bit.ly/3VNEj6I
8. Aprea Therapeutics reports third quarter 2022 financial results and provides update on business operations. News release. Aprea Therapeutics. Accessed November 9, 2022. November 29, 2022. https://bit.ly/3gTllg9
9. Kikushige Y, Miyamoto T. Identification of TIM-3 as a leukemic stem cell surface molecule in primary acute myeloid leukemia. Oncology. 2015;89(suppl 1):28-32. doi:10.1159/000431062
10. Brunner AM, Esteve J, Porkka K, et al. Effi cacy and safety of sabatolimab (MBG453) in combination with hypometh-ylating agents (HMAs) in patients (Pts) with very high/high-risk myelodysplastic syndrome (vHR/HR-MDS) and acute myeloid leukemia (AML): final analysis from a phase Ib study. Presented at: 63rd American Society of Hematology Annual Meeting and Exposition; November 5, 2021; Atlanta, GA. Abstract 637.
11. Daver NG, Dail M, Garcia JS, et al. Venetoclax and idasanutlin in relapsed/refractory AML: a non-randomized, open-label phase 1b trial. Blood. Published online October 202, 2022. doi:10.1182/blood.2022016362
12. Konopleva MY, Röllig C, Cavenagh J, et al. Idasanutlin plus cytarabine in relapsed or refractory acute myeloid leukemia: results of the MIRROS trial. Blood Adv. 2022;6(14):4147-4156. doi:10.1182/bloodadvances.2021006303
13. Sekiguchi N, Kasahara S, Miyamoto T, et al. Phase I dose-escalation study of milademetan in patients with re-lapsed or refractory acute myeloid leukemia. Int J Hematol. Published online October 19, 2022. doi:10.1007/s12185-022-03464-z
14. Stein EM, DeAngelo DJ, Chromik J, et al. Results from a first-in-human phase I study of siremadlin (HDM201) in patients with advanced wild-type TP53 solid tumors and acute leukemia. Clin Cancer Res. 2022;28(5):870-881. doi:10.1158/1078-0432.CCR-21-1295
15. Guy DG, Uy GL. Bispecific antibodies for the treatment of acute myeloid leukemia. Curr Hematol Malig Rep. 2018;13(6):417-425. doi:10.1007/s11899-018-0472-8
16. Uy GL, Aldoss I, Foster MC, et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood. 2021;137(6):751-762. doi:10.1182/blood.2020007732
17. NextCure to present trial in progress poster for NC525 at the 2022 American Society of Hematology (ASH) Annual Meeting. NextCure. November 3, 2022. Accessed November 29, 2022. https://bit.ly/3VNBum4

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More