Investigators Assemble to Define Cellular Therapy’s Earlier Role in Blood Cancers
Andre H. Goy, MD
Multidisciplinary communication is needed to make sense of the latest advances, said Andre H. Goy, MD, physician in chief at Hackensack Meridian Health Oncology Care Transformation Service, and chairman & chief physician offi cer at John Theurer Cancer Center. In an interview with Targeted Therapies in Oncology™, Goy, who is program chair of the upcoming 27th Annual International Congress on Hematologic Malignancies®: Focus on Leukemias, Lymphomas, and Myeloma, sponsored by Physicians’ Education Resource (PER®), LLC. He hopes the conference will address 2 important real-world questions: For the patient with a hematologic malignancy, what are the best treatment options now? And for clinicians, what is the best sequence of care that they can provide patients to optimize therapy?
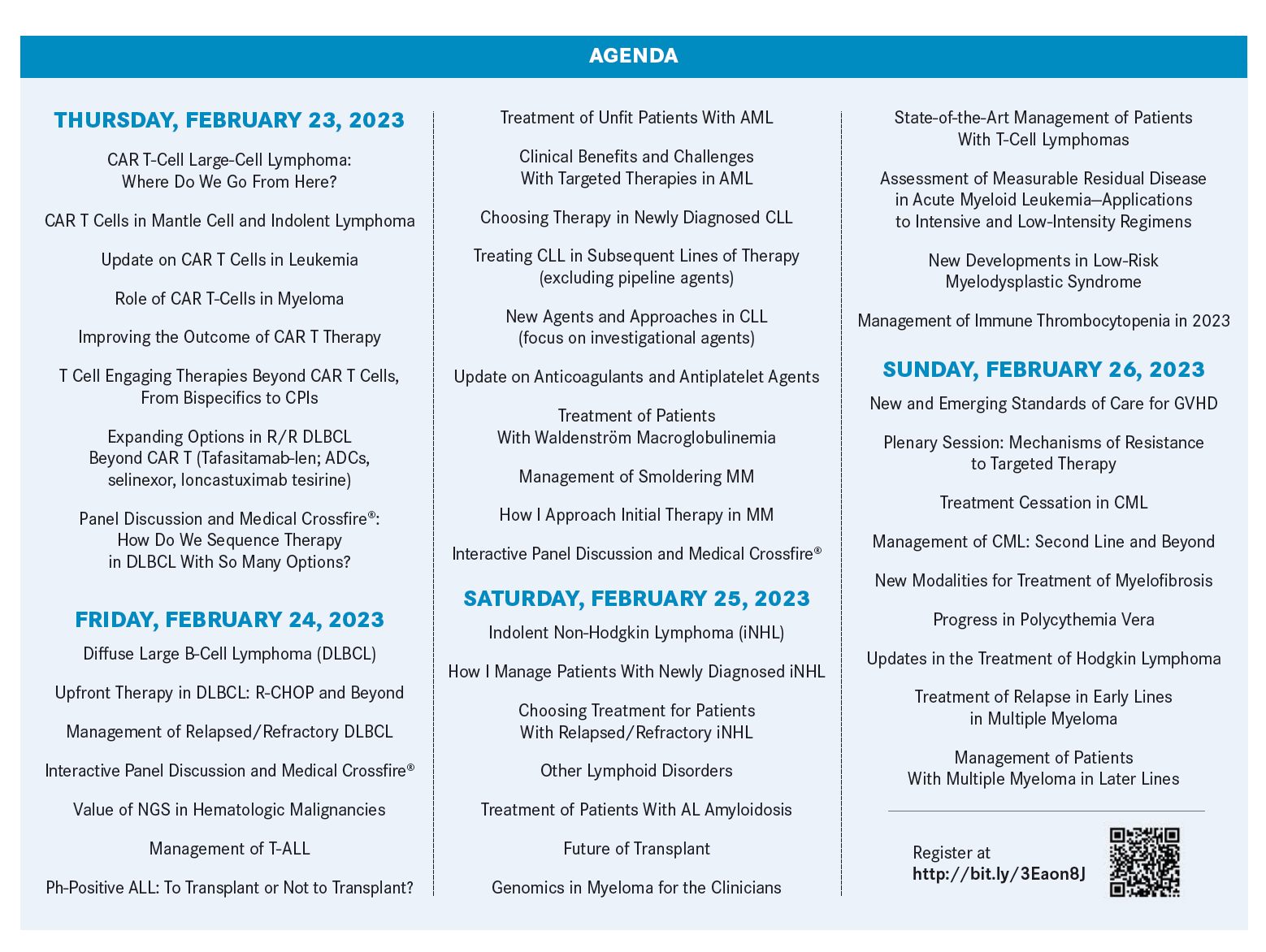
The conference, February 23 to 26, 2023, in Miami Beach, Florida, is being held on the heels of the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, December 10 to 13, 2022, in New Orleans, Louisiana.
“The new data presented at ASH can change how we treat patients, and a goal of our conference will be figuring out how to put those changes into practice,” said Goy, who is also the Lydia Pfund Chair for Lymphoma, academic chairman oncology at Hackensack Meridian School of Medicine, and professor of medicine, Georgetown University.
Mantle Cell Lymphoma
Turning to his area of expertise in mantle cell lymphoma (MCL), Goy noted the shifting treatment pardigm as novel agents are incorporated into standard regimens and next-generation sequencing (NGS) gains a foothold. This shift allows clinicians and investigators to identify, beyond clinical prognostic models, the distinct features that can predict poor outcomes in conventional approaches.
Recognizing the molecular diversity of MCL and the development of Bruton tyrosine kinase (BTK) inhibitors has moved the fi eld forward. “We have 6 drugs, including BTK inhibitors, lenalidomide (Revlimid), bortezomib (Velcade), and now chimeric antigen receptor (CAR) T-cell therapies that are game changers [in the MCL space],” Goy said.
There are now clearly recognized subtypes of the disease. These include the indolent cases, which can remain stable for years. At the other end of the spectrum, some patients have aggressive blastoid disease. These patients have TP53 mutations, frequently complex karyotypes, and a high proliferative rate. “They often do poorly when treated with standard therapy,” Goy said.
The majority of cases of MCL fall somewhere in between these 2 subtypes, and are often referred to as “smoldering MCL.” These patients can be asymptomatic with low tumor burden.
For patients with smoldering MCL, or for patients who are stem cell transplant–eligible, a treatment option for consideration includes a chemoimmunotherapy regimen of rituximab, an anti-CD20 monoclonal antibody, and cytarabine-containing regimen, followed by transplant and maintenance with rituximab.
Targeted Approaches
A targeted approach as a key component of either a doublet or triplet regimen has provided the opportunity to minimize chemotherapy exposure, Goy said. In the TRIANGLE trial (NCT02858258), the addition of ibrutinib (Imbruvica) to induction and as maintenance therapy with or without transplant improved outcomes.2
The WINDOW-1 trial (NCT02427620) evaluated induction with ibrutinib and rituximab. Investigators assessed whether the upfront use of targeted therapies could result in remission without chemotherapy and allow for consolidation with only 4 cycles of chemotherapy.3 The trial demonstrated that the use of targeted therapies was safe and active with a complete response (CR) rate of 90% in patients who had received ibrutinib and rituximab alone.
Adding venetoclax (Venclexta) to ibrutinib/ rituximab, followed by risk-stratifi ed observation or a short course of rituximab, hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine, demonstrated that a triplet chemotherapy-free induction could reduce toxicity and complications in patients with MCL.4
Fifty patients were enrolled in the phase 2 WINDOW-II trial (NCT03710772). In part 1 of the trial, patients received ibrutinib plus rituximab for 4 cycles. After part 2 consolidation, patients received the triplet regimen for 2 years. The median progression-free survival (PFS) and overall survival (OS) were not reached (2 years, 92% and 90%, respectively). The median PFS and OS were inferior in patients with blastoid/pleomorphic MCL compared with patients with classic MCL (P = .01 and P = .03, respectively).4
In aggressive lymphomas, CAR agents have been approved in multiple indications. In MCL specifi cally, brexucabtagene autoleucel (Tecartus) was shown to have had an overall response rate greater than 90% and a CR rate greater than 60% in heavily pretreated patients with MCL.5,6
In the ZUMA-2 trial (NCT02601313), at a median follow-up of 12.3 months (range, 7.0-32.3), 57% of the 60 patients in the primary efficacy analysis were in remission. At 12 months, the estimated PFS and OS rates were 61% and 83%, respectively. Investigators reported that durable remissions were observed in the majority of patients with relapsed or refractory MCL. Toxicities and safety signals were consistent with those reported for other CAR T-cell agents.
Looking Ahead
Looking ahead, Goy is encouraged by the findings involving CAR T-cell therapies. The role of CAR T-cell therapy in leukemia, myeloma, lymphoma, mantle cell lymphoma, and diffuse large B-celly lymphoma will be covered on the first day of the conference.
REFERENCES
1. Hanel W, Epperla N. Emerging therapies in mantle cell lymphoma. J Hematol Oncol. 2020;13(1):79. doi:10.1186/s13045-020-00914-1
2. Dreyling M, Ladetto M, Doorduijn JK, et al. Triangle: autologous transplantation after a rituximab/ibrutinib/ara-c containing induction in generalized mantle cell lymphoma -a randomized European MCL Network Trial. Blood.2019;134(suppl1):2816. doi:10.1182/blood-2019-127863
3. Wang ML, Jain P, Zhao S, et al. Ibrutinib-rituximab followed by R-HCVAD as frontline treatment for young patients (≤65 years) with mantle cell lymphoma (WINDOW-1): a single-arm, phase 2 trial. Lancet Oncol. 2022;23(3):406-415. doi:10.1016/S1470-2045(21)00638-0
4. Wang M, Jain P, Lee HJ, et al. Ibrutinib plus rituximab and venetoclax (IRV) followed by risk-stratified observation or short course R-hypercvad/MTX in young patients with previously untreated mantle cell lymphoma -phase-II Window-2 clinical trial. Blood. 2021;138(suppl 1):3525. doi:10.1182/blood-2021-153390
5. CAR T-cell therapy approved by FDA for mantle cell lymphoma. Cancer Currentsblog. August 24, 2020. Accessed October 21, 2022. https://bit.ly/3gmyrlf
6. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331-1342. doi:10.1056/NEJMoa1914347

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More