Immune Checkpoint Approaches in AML and MDS: A Next Frontier?
The researchers discuss the rationale for checkpoint-based therapies including antibodies to PD-1/PD-L1 and CTLA-4, an overview of clinical experience with these agents, and future considerations and combinations of these agents.
Lucia Masarova, MD
Abstract
Therapy for acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) has changed modestly in the past 4 decades. The prognosis for AML and MDS remains poor, especially in patients with adverse prognostic factors, older age, or relapsed disease. An increased understanding of tumor biology and immune regulation has led to the development of novel molecular, epigenetic, and immune therapies for these patients. This review focuses on immunotherapeutic approaches in AML and MDS, with a special focus on the rationale for checkpoint-based therapies including antibodies to programmed cell-death protein-1 (PD-1/ PD-L1) and cytotoxic T-lymphocyteassociated antigen 4 (CTLA-4), an overview of clinical experience with these agents, and future considerations and combinations of these agents. Overexpression of various checkpoint receptors on T cells and ligands on AML blasts, especially those regulating the major inhibitory checkpoint pathways such as CTLA-4 and PD-1, interferes with effective T-cell antitumor response and is associated with leukemic progression in preclinical models. Novel monoclonal antibodies targeting CTLA-4 (eg, ipilimumab) or PD-1/PD-L1 (eg, nivolumab, pembrolizumab, and atezolizumab) can reverse immune suppression by unleashing the patient’s T cells to attack leukemic cells. Results of numerous, ongoing early clinical trials evaluating these agents in monotherapy or in combination with the hypomethylating agents in relapsed AML and frontline posthypomethylating MDS are showing promising activity and safety. A phase I clinical trial with ipilimumab showed disease stabilization in 45% of patients with high-risk MDS. Ipilimumab also produced complete responses in 42% of patients with previously heavily treated AML who relapsed after stem cell transplantation in a phase I trial. A phase I/II trial with nivolumab, in combination with hypomethylating agent azacitidine, in patients with relapsed AML (median 2 prior therapies), demonstrated an overall response rate of 34%, lower early mortality, and the responses appear to be durable. These clinical trials will likely help us answer a number of critical issues regarding the utility of immune checkpoint therapy in AML and MDS, including the optimal dose and schedule of these agents, the efficacy and safety when used as a monotherapy or in combinations, the ideal agents to combine in the next wave of immune–immune or immune–molecular combinations, the management of checkpoint specific toxicities, and the identification and implementation of baseline and dynamic biomarkers of response to best select patients suited for these modalities.
Hagop Kantarjian, MD
Naval Daver, MD
Treatment for acute myeloid leukemia (AML), the most prevalent acute leukemia among adults, has improved modestly over the last 4 decades. Standard frontline treatment (“7+3”), introduced in the 1970s1, provides long-term overall survival (OS) in 40% of young adults. Outcomes of older patients, patients with adverse karyotype or TP53 mutation, or relapsed disease remain very poor with <10% long-term survivors.2-4A number of new molecular and epigenetic therapies have shown positive results in phase II/III studies and will likely change our current approaches to AML therapy in the near future.5-6Beyond molecular therapies, in the last year, immune therapies have emerged as an area of intense investigation in AML and myelodysplastic syndrome (MDS). The significant role of the immune system in producing durable remissions in hematologic malignancies has long been demonstrated by the success of allogeneic stem cell transplantation (aSCT) in producing long-term remissions in patients with leukemia, lymphoma, and multiple myeloma.7A better understanding of cancer immunology and availability of improved therapeutic tools including checkpoint antibodies, chimeric antigen receptor (CAR)-T cells, vaccines, and adoptive T-cell approaches, coupled with the success of these therapies in solid (melanoma, lung, head, and neck cancers) and liquid (Hodgkin lymphoma, multiple myeloma) tumors in recent years, has further fueled efforts to explore the efficacy, tolerability, and optimal approach to using these therapies in hematologic malignancies including AML, MDS, myelo brosis (MF), and chronic lymphocytic leukemia (CLL).
Immune homeostasis is normally maintained by balanced interactions between numerous costimulatory and coinhibitory signals. Tumor-mediated deregulation of T-cell responses leads to evasion by tumor cells of T-cell immune response.8The best- known receptors regulating T-cell activation or inhibition are the co-stimulatory receptors CD28, 4-1BB, CD27, CD80, and CD86, and co-inhibitory receptors cytotoxic T-lymphocyte-associated-protein 4 (CTLA- 4) and programmed cell-death protein (PD-1) with its ligands (PD-L1, PD-L2), respectively. Since the discovery of the inhibitory CTLA-4 receptor on the surface of T cells and the ability to unleash T cells by blocking the inhibitory CTLA-4 receptor,9a large number of clinical trials with checkpoint inhibitors, mainly targeting CTLA-4, PD-1 or PD-L1, or both receptors, have been conducted in solid tumors. CTLA- 4 is expressed predominantly on the T cells in the lymph nodes and regulates early T-cell activation, primarily by downregulation of T-helper cells and upregulation of T-regulatory cells,9, 10whereas the PD-1 pathway regulates in ammatory responses in the peripheral tissues by inhibiting effector T cells.11(Figure 1). The fact that CTLA-4 and PD-1/ PD-L1 work at different levels in the immune system likely explains the demonstrated synergy when these agents are combined (Figure 2).12
The evaluation of immunotherapy in leukemia is supported by the observation that various clinically targetable checkpoint receptors and ligands are overexpressed on T cells in ltrating the leukemic microenvironment, as opposed to T cells in the bone marrow aspirates of healthy individuals.13Daver et al evaluated 74 AML patients (36 untreated AML, 38 relapsed AML) to identify the degree and nature of T-cell infiltrate and the expression of a variety of checkpoint receptors and ligands on the T-lym- phocytes and blasts, respectively, in the bone marrow and peripheral blood in patients with AML, as compared with healthy donors.14Among a variety of checkpoint receptors evaluated, they noted that the bone marrow aspirates from patients with AML had significantly more PD-1positive T cells (including increased PD-1 CD8+, PD-1 CD4+FoxP3+CD127- negative, and CD4+FoxP3-CD127lo/+ T cells) as compared with healthy donors. The other checkpoint of interest that was identified was OX40, wherein bone marrow aspirates in patients with AML had significantly increased OX40-postive T cells. It has been reported that overexpression of PD-1 and CTLA-4 is associated with more aggressive leukemia15and that PD-1 expression is increased at progression from MDS to AML or AML relapse. PD-1 blockade to enhance the antileukemia response, reduce leukemia organ infiltration and increase survival in murine models of AML.16, 17
A phase I study of the PD-1 inhibitor pidilizumab in hematological malignancies including 9 patients with AML/MDS, showed safety, yet minimal response as a single agent with only 1 patient achieving a stable disease response.18CTLA-4 inhibitor ipilimumab showed disease stabilization in 45% of patients with high-risk MDS in a phase I study (5 of 11), 4 of whom had a durable response >6 months. Grade 3 immune related adverse events (AEs) were noticed in 7 patients (63%) and were easily managed with steroids.19A recent study highlighted the ef cacy of ipilimumab as a single agent in post allogeneic stem cell transplant (post-ASCT) relapsed AML. Twenty- eight patients were treated, including 12 patients with relapsed AML (including 3 patients with leukemia cutis). Complete responses were seen in 5 (42%) patients with AML, including 3 patients with leukemia cutis, and 4 patients who had durable responses for >1 year. This compares favorably to historical outcomes with post-ASCT relapsed AML, where expected response rates are 15% to 20% and <10% are expected to be alive at 1 year. Immune-related AEs were observed in 21% of patients (n = 6), including 1 death and graft versus host disease precluding further administration of ipilimumab in 14% (n = 4).20
Epigenetic agents are a standard therapy for front-line and salvage in patients with AML and MDS.21, 22The epigenetic agent, azacitidine, has been shown to upregulate PD-1, PD-L1, and PD-L2 (≥2-fold) in >50% of 61 evaluable patients with AML/MDS, which may be associated with emergence of resistance to azacitidine.23Therefore, concomitant inhibition of the PD-1/PD-L1 axis may prevent or overcome resistance. Trials combining epigenetic agents with PD-1/PD-L1 based therapies have recently started enrollment for AML and/or MDS including azacitidine with the PD-1 inhibitor nivolumab (NCT02397720), azacitidine with or without PD-L1 inhibitor durvalumab (NCT02775903), azacitidine with or without the PD-L1 inhibitor atezolizumab (NCT02508870). Results from the first to open of these studies, a phase IB/II trial of nivolumab with 5-azacytidine, are encouraging. In an interim analysis, 35 evaluable patients with a median follow-up of 3.6 months showed an overall response rate of 34% including 6 complete remissions and/or complete remission with insuficient count recovery (CR/ CRi);(18%); 5 hematologic improvements including 2 patients who had concomitant >50% blast reduc- tion (16%).24This compares favorably with a historic response rate of 15% to 20% in a similar patient treated with azacitidine alone or decitabine alone.
Additionally, 5 patients (23%) had a >50% blast reduction and 2 had stable disease (7%). The patients had a significantly improved overall response rate, 8-week mortality, and median progression-free survival as compared with historical outcomes with azacitidine-based salvage protocols from the same institution, but the follow-up at this time is short. Grade 2 to 4 immune adverse events (iAEs) were observed in 11 (31%) patients with a time to onset of 4 days to 3.5 months. The pattern of iAEs was different from that seen in solid tumors with most common iAEs of pneumonitis, nephritis, colitis, and dermatitis as opposed to endocrine, skin, and liver iAEs typically seen in solid tumors. Also different from solid tumor experiences, all iAEs responded quickly to steroids with successful rechallenge with nivolumab in all, but one case.24
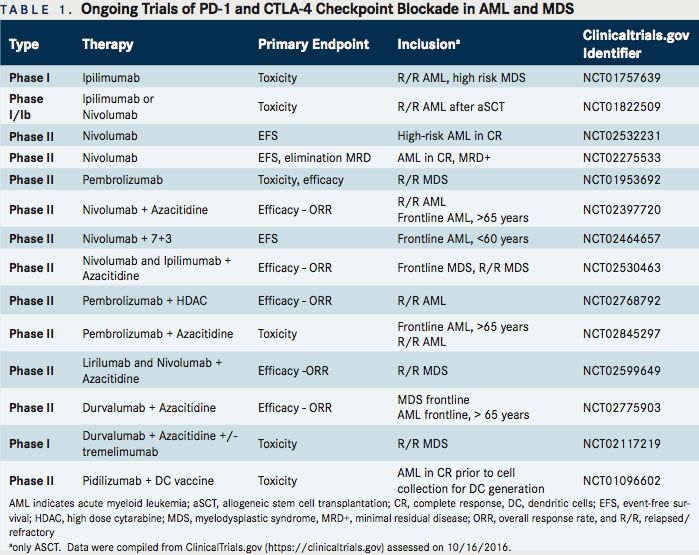
Table 1 provides details on currently ongoing combinational trials of PD-1/CTLA-4 inhibitors with epigenetic, cytotoxic agents, or tumor specific vaccines for AML and MDS.
In summary, emerging data suggest potential efficacy of checkpoint inhibitors in AML, especially in combination with hypomethylating agents. A number of trials evaluating these combinations and comparing their efficacy and tolerability to a single hypomethylating agent in both AML and MDS have been initiated and will help us better answer these questions. Other immune-based approaches for patients with AML/MDS, include conjugated antibodies targeted to leukemia specific antigens that deliver toxic payload to leukemic cells (SGN 33A, SGN 123, IMGNCD33 and CD123), bispecific T-cell engaging antibodies (AMG CD3 CD33, JNJ CD3 CD123, XmAb CD3 CD123), DARTs, and CAR T-based approaches targeting CD123 or CLEC12A. These agents may eventually be rational choices to combine with checkpoint inhibitors to develop immuneimmune strategies for AML and MDS or in combination with epigenetic and cytotoxic therapies. The ongoing clinical trials will likely help answer a number of critical issues regarding the utility of immunotherapy in leukemia including accurate timing and dynamics of response to such therapies, identification of additional novel checkpoint targets in AML, biomarkers to allow for selection of candidates with highest propensity to response, awareness and management of immune toxicities, and mechanisms of immune modulation and resistance.
References:
- Yates JW, Wallace HJ, Jr., Ellison RR, Holland JF. Cytosine arabinoside (NSC- 63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57(4):485-488.
- Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106(5):1090-1098.
- Burnett AK, Hills RK, Milligan D, et al. Identi cation of patients with acute myeloblastic leukemia who bene t from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369-377. doi: 10.1200/ JCO.2010.31.4310.
- Kadia TM, Jain P, Ravandi F, et al. TP53 mutations in newly diagnosed acute myeloid leukemia: Clinicomolecular characteristics, response to therapy, and outcomes. Cancer. 2016. doi: 10.1002/cncr.30203.
- Daver N, Cortes J, Kantarjian H, Ravandi F. Acute myeloid leukemia: advancing clinicaltrialsandpromisingtherapeutics.ExpertRevHematol.2016;9(5):433-445. doi: 10.1586/17474086.2016.1158096.
- Kantarjian H. Acute myeloid leukemia major progress over four decades and glimpses into the future. Am J Hematol. 2016;91(1):131-145. doi: 10.1002/ajh.24246.
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bonemarrowtransplantation.Blood.1990;75(3):555-562.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. doi: 10.1038/nrc3239.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA- 4blockade.Science.1996;271(5256):1734-1736.
- Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1(9):793-801.
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif- carrying immunoreceptor. Immunity. 1999;11(2):141-151.
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214. doi:10.1016/j.cell.2015.03.030.
- Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17(13):4232-4244. doi: 10.1158/1078-0432.CCR-10-2660.
- DaverNBS,Garcia-ManeroG,CortesJ,etal.De ningtheimmunecheckpoint landscape in patients with acute myeloid leukemia. Poster presented at: 58th Annual Meeting and Exposition American Society of Hematology; December 4, 2016; San Diego, CA. https://ash.confex.com/ash/2016/webprogram/ Paper97460.html. Accessed January 3, 2017.
- Fevery S, Billiau AD, Sprangers B, et al. CTLA-4 blockade in murine bone marrow chimeras induces a host-derived antileukemic e ect without graft-versus-host disease. Leukemia. 2007, 21(7):1451-1459.
- Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114(8):1545- 1552. doi: 10.1182/blood-2009-03-206672.
- Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim-3 and PD-1 identi es a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood.2011;117(17):4501-4510. doi: 10.1182/ blood-2010-10-310425.
- Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044-3051. doi: 10.1158/1078-0432.CCR-07-4079.
- Zeidan AM, Zeidner, JF, Du eld A, et al. Stabilization of myelodysplastic syndromes (MDS) following hypomethylating agent (HMAs) failure using the immune checkpoint inhibitor ipilimumab: a phase I trial. Poster presented at: 58th Annual Meeting and Exposition American Society of Hematology; December 5, 2016; San Diego, CA. https://ash.confex.com/ash/2015/webprogramscheduler/ Paper82111.html. Accessed on January 3, 2017.
- Davids MS, Kim HT, Bachireddy P, et al; Leukemia and Lymphoma Society Blood Cancer Research Partnership. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375(2):143-153. doi: 10.1056/ NEJMoa1601202.
- KantarjianHM,ThomasXG,DmoszynskaA,etal.Multicenter,randomized,open- label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670- 2677. doi: 10.1200/JCO.2011.38.9429.
- DombretH,SeymourJF,ButrymA,etal.Internationalphase3studyof azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299. doi: 10.1182/ blood-2015-01-621664.
- YangH,Bueso-RamosC,DiNardoC,etal.ExpressionofPD-L1,PD-L2,PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280-1288. doi: 10.1038/ leu.2013.355.
- Daver N BS, Garcia-Manero G, Cortes J, et al. Phase IB/II study of nivolumab in combination with 5-azacytidine in patients with relapsed acute myeloid leukemia. Poster presented at: 58th Annual Meeting and Exposition American Society of Hematology; December 5, 2016; San Diego, CA. https://ash.confex.com/ ash/2016/webprogram/Paper98062.html. Accessed January 3, 2017.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More