Immune Checkpoint Inhibitors in CRC
Ali Maawy, MD, and Patrick M. Boland, MD, provide a brief review of the immune environment of colorectal cancer (CRC) and recently reported studies of immunotherapy in CRC.
Patrick M. Boland, MD
Abstract
Immune checkpoint inhibitors targeting the cytotoxic T-lymphocyte associated protein-4 (CTLA-4) and programmed cell death 1 (PD-1) receptors have recently made their way into the oncology clinics, producing substantial clinical benefits for certain tumor types and patients. Data are rapidly emerging for multiple malignancies, with numerous additional molecules and antibodies in development. Considering advanced colorectal cancer, the early signals of activity are largely involving PD-1 inhibition, specifically against a subset of colorectal cancer (CRC), microsatellite instability high (MSI-H) tumors. This has prompted multiple studies and an even stronger push for universal MSI testing. Unfortunately, the colon cancers which are not MSI-H (MSS), those which make up the majority of the tumors in clinical practice, have not seen benefit through single agent PD-1/PD-L1 inhibition. Recently, a combination of MEK inhibition and PD-L1 inhibition demonstrated highly intriguing, but preliminary evidence of clinical efficacy, prompting the initiation of a phase III trial (NCT02788279). Numerous additional efforts are underway evaluating immunomodulatory therapeutic combinations that may favorably alter the tumor microenvironment. This article presents a brief review of the immune environment of CRC, recently reported studies of immunotherapy in CRC, and a survey of ongoing efforts.
Introduction
Largely attributable to the development of immune checkpoint inhibitors, specifically monoclonal antibodies targeting cytotoxic T-lymphochyte associated protein-4 (CTLA-4), programmed cell death (PD-1), or PD-L1, there has recently been an ‘immunotherapy revolution’ in the oncology community. PD-1 is ubiq- uitously expressed in T cells, B cells and NK cells and is similarly upregulated in an inflammatory milieu. PD-1 binds to its ligands PD-L1 and PD-L2 and induces T effector cell exhaustion and conversion of T effector cells to regulatory T (Treg) cells. PD-L1 is expressed in multiple tissues, including tumor cells and antigen presenting cells, while PD-L2 is selectively expressed on hematopoietic cells.1,2Tumors that have not been traditionally considered immune responsive have now been shown to be susceptible to immune modulation of the tumor microenvironment. Conclusive benefit has been demonstrated in nonsmall cell lung cancer (NSCLC), melanoma, renal cell cancer, urothelial bladder cancer, and head and neck cancer among others, resulting in approval of multiple PD-1 and/or PD-L1 inhibitors by the FDA. In the meantime, intriguing results are emerging in multiple other tumor types; recently, benefit has been demonstrated in mismatch repair (MMR)-deficient (or microsatellite instability high [MSI-H]) colorectal cancer (CRC).
Ali Maawy, MD
A hallmark of neoplastic transformation and subsequent tumor survival in the host milieu is immune evasion. The prevailing mechanism by which this occurs is termed cancer immunoediting, a process involving 3 phases.3In the initial phase, there is activation of the adaptive and innate immune response to tumor neoantigens, characterized by trafficking of NK cells, along with B and T lymphocytes. This phase is also associated with secretion of cytokines: IFN-α, IFN-γ, TNF, and IL-12. If the neoplastic tissue survives this, an equilibrium phase follows, involving a balance between tumor cell destruction by the adaptive immune system, namely activated CD4+ and CD8+ cells. Here, malignant clones persist but an active immune response prevents overall tumor growth and produces dormancy. Ultimately, the clones are able to adapt and escape, thus resulting in immune evasion.
Immunologic escape can occur via multiple mechanisms, including loss of antigen presenting ability, release of inhibitory cytokines, and expression of immune checkpoint molecules such as PD-1 and PD-L1 that would serve to suppress effector T- cell activity.4,5In addition, many signaling pathways that are believed to be drivers of oncogenesis have been noted to have secondary immune modulating effects. Activation of the WNT/β-catenin pathway in metastatic melanoma is correlated with an absence of T cells in the tumor microenvironment.6This is of great relevance, as this pathway is activated in the majority of CRCs.
Immunity and Colorectal Cancer
CRCs are believed to develop through a stepwise process of mutations in key genes such as APC, KRAS, P53, and SMAD4, first delineated more than 2 decades ago.7,8Genetic alterations have been firmly linked to tumorigenesis for many years. Immune evasion was not part of the originally proposed 6 hallmarks of cancer, but it has now been added to this list as a core feature of tumorigenesis.9Inflammation, on the other hand, has been long associated with cancer, with a link to chronic inflammatory conditions that appear to facilitate neoplastic transformation, as with inflammatory bowel disease and CRC. In fact, epidemiologic data and some randomized data support a role for nonsteroidal anti-inflammatory drugs (NSAIDs) in decreasing the formation of both polyps and CRCs.10,11These points are raised to highlight the delicate interplay between immune activation, the healthy body, and cancer. Disruption of the delicate balance of the immune system, hyperactivation, inappropriate activation, or immunosuppression can all lead to development of cancer.
About a decade ago, evidence came to light suggesting that subsets of effector immune cells are important in CRC. Using markers to evaluate numbers of all T cells (CD3+), cytotoxic T cells (CD8+), and memory T cells (CD45RO+) in the tumor core as well as the invasive margin, Galon et al demonstrated superior outcomes for those patients with tumors containing more dense T-cell infltration, compared with those which were relatively T-cell depleted.12In fact, both total T cells (CD3) and memory T cells (CD45RO+) proved superior to TNM staging in predicting outcomes. Multiple other studies have evaluated the predictive capacity of lymphocyte subsets in CRC, with a more robust inflammatory infiltrate generally conferring a more favorable prognosis.13Recently, the Immunoscore methodology, which assesses CD3+ and CD8+ cellular density in various compartments, has been demonstrated to be predictive of response as well as prognosis in locally advanced rectal adenocarcinomas undergoing neoadjuvant chemoradiation.14Thus, immune activation and immune effector subsets are important on their own, but also potentially relevant in the efficacy of standard anticancer therapies.
MSI and Colorectal Cancer
At the most basic, clinically relevant level, CRC can be segregated by site of origin, by RAS and BRAF mutational status, and by microsatellite status (MSI- H or microsatellite stable [MSS]). Microsatellite instability is seen in 15% to 20% of early-stage CRCs and in 3% of metastatic CRCs.15These tumors are more commonly right-sided, mucinous, poorly differentiated, and characterized by lymphocytic infiltration. They also more commonly possess BRAF mutations. The observed MSI is due to infidelity of DNA replication, owing to dysfunction of one of the MMR proteins (MLH1, MSH2, MSH6, PMS2), in line with the other common terminology: deficient mismatch repair (dMMR). While MSI/dMMR has been linked to Lynch syndrome, it is important to note that MSI-H status is most often due to an epigenetic alteration (hypermethylation of the MLH1 promoter), which silences the MLH1 protein. Epigenetic MLH1 silencing represents approximately 80% of all MSI-H CRCs, usually sporadically occurring in the patient. This underscores an important point: most MSI-H tumors are not Lynch syndrome related.16
Initially, MSI testing emerged as a means to screen for Lynch syndrome, although it was later found to have prognostic and predictive importance.17Patients with early-stage CRC and MSI-H tumors appear to have improved prognoses, whereas those with stage IV MSI-H tumors may fare worse.15,17It has been further demonstrated that patients with stage II MSI-H CRC treated with uorouracil (5-FU)- based therapy derive little to no benefit. In fact, such patients potentially face worse outcomes if their tumors are MSI-H, compared with MSS.18Further retrospective data suggest that the addition of oxaliplatin may overcome this observed resistance to 5-FU alone, of relevance for stage III CRC.19
Importantly, CRCs with MSI-H are characterized by greatly elevated mutational rates. It is widely believed that the resultant mutations may serve as tumor neoantigens, triggering an immune response. Recent data suggest a correlation between frameshift mutation frequency and the density of tumor-infiltrating CD8+ T cells, supporting this hypothesis that the mutational landscape is directly related to tumor antigenicity.20Finally, it is worth noting that MSI-H tumors, which would otherwise seem to exist in a microenvironment that is more innately hostile to neoplasms, are characterized by highly upregulated expression of multiple immune checkpoints, including PD-1, PD-L1, CTLA-4, LAG-3, and IDO.21This implies that MSI-H CRCs are a tumor type characterized by a robust population of immune effector cells, but also possessing an immune exhaustion phenotype within the microenvironment.
Immune Checkpoint Inhibitors in Colorectal Cancer
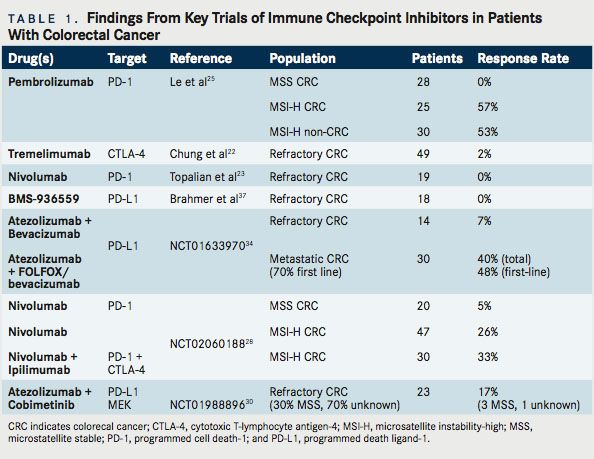
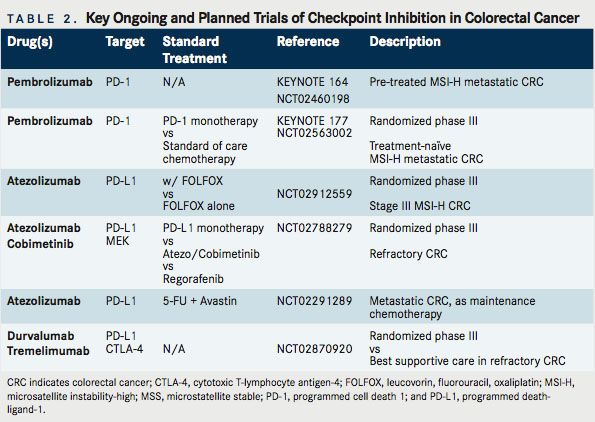
As noted, immunotherapy is not a new concept in CRC. Multiple previous efforts have been undertaken. CRC, too, has been the subject of studies involving CTLA-4, PD-1, and PD-L1 inhibition. Relevant completed studies and those with informative interim analyses are described in Table 1. Key planned trials are detailed in Table 2. A narrative of the developments follows.
CTLA-4 Inhibitor Monotherapy
Tremelimumab
Tremelimumab is a fully human immunoglobulin G2 (IgG2) monoclonal antibody (mAb) that binds to the CTLA-4 receptor. Because of its initial activity in melanoma, as well as supporting preclinical data, a phase 2 study of tremelimumab was undertaken in patients with refractory metastatic CRC, at a dose of 15 mg/kg every 90 days. In this multicenter study, 49 patients with chemorefractory metastatic CRC were treated, with a median duration on study of 2.3 months. The 6-month progression-free survival (PFS) was a meager 2.1%. The sole patient who received more than 1 dose of therapy experienced a partial response by Response Evaluation Criteria In Solid Tumors (RECIST), continuing without disease progression for 14.5 months and surviving for 19 months from the start of therapy. Toxicities were as expected, with an 11% incidence of grade 3 diarrhea.22Although 1 patient appeared to derive substantial benefit, the limited response rate did not suggest general applicability. As such, this therapy has not been further pursued. Unfortunately, the tumor or host characteristics that enabled the responding patient to benefit have not emerged to date.
PD-1/PD-L1 Inhibitor Monotherapy
Nivolumab
Nivolumab is a fully humanized IgG4 mAb that binds and inhibits PD-1. Nivolumab (Opdivo) garnered broad attention when the initial phase 1 data were presented in 2012.23In the study, 296 patients were treated at various doses (1 mg/kg, 3 mg/kg,10 mg/ kg) every 2 weeks. Responses were noted in melanoma (28%), NSCLC (18%), and RCC (27%). Responses were durable, with up to 1 year or more in 20 of 31 initially responding patients. No clear doseresponse relationship was demonstrated. In 42 tumor samples that underwent immunohistochemical analysis for PD-L1 expression, a 36% objective response was noted in PD-L1–positive tumors and 0% response rate in PD-L1–negative tumors. In this study, 17 patients had prostate cancer and 19 patients had CRC. No objective responses were observed in either cohort.
A later report describing several patients from the initial nivolumab study was published in 2013. That study noted that 1 patient with CRC experienced a complete response (CR) to therapy, ongoing at 3 years. This patient was a 71-year-old male with MSI- H CRC, refractory to prior treatment, and notably with membranous PD-L1 expression on tumor-infiltrating macrophages and lymphocytes as well as rare tumor cells associated with in ltrating PD-1+ and CD-3+ T cells.24Thus, significant benefit was possible with this drug, but only in the rare patient.
Pembrolizumab
Pembrolizumab (Keytruda) is an IgG4 mAb that binds and inhibits PD-1. Pembrolizumab has received great attention recently in CRC, attributable to the highly encouraging results of a multicenter phase II study initially presented at the 2015 ASCO Annual Meeting. In this study, patients with chemo-refractory MSI-H or MSS metastatic CRC, as well as MSI-H cancers of other origin, were included. The trial was based on the rationale described above: previous activity in 1 patient with MSI-H CRC, elevated immunogenic tumor neoantigens, and the inflamed tumor environment often seen in MSI-H cancers. A standardized polymerase chain reaction (PCR)- based test was used to assess MSI, with pembrolizumab was administered every 2 weeks at a dose of 10 mg/kg.25At the time of the initial presentation, no responses were recorded among the mismatch repair (MMR) proficient (pMMR or MSS) CRC patients, with 2 (11%) achieving stable disease at 12 weeks. However, of the 10 evaluable MSI-H patients with CRC, 4 (40%) experienced a re- sponse, with 7 of 9 (78%) patients achieving stable disease. In parallel, 5 (71%) of the 7 evaluable MSI-H patients with tumors of non-CRC origin experienced a response, with 1 (14%) achieving a CR.25
Updated results were presented at the 2016 ASCO Annual Meeting. There were 83 evaluable patients with refractory tumors described: MSS CRC (28 patients), MSI-H CRC (25 patients), and MSI-H cancers of other origin (30 patients). Among the MSI-H patients with CRC, 16 (57%) experienced a response, with 3 (11%) having a CR and 9 (32%) patients achieving stable disease. Median (PFS) was not reached (NR) for MSI-H CRC and 2.4 months for MSS CRC (HR, 0.135; 95% CI, 0.043-0.191; P ≤.0001). Median overall survival (OS) was NR for MSI-H CRC versus 6 months for MSS CRC (HR,0.247;95%CI,0.117-0.589;P=.001).For MSI-H CRC, the PFS rate was 61% at 24 months and the OS rate was 66% at 24 months. Similarly, among patients with non-CRC MSI-H tumors, 16 (53%) experienced a response, with 9 (30%) patients achieving a CR; a pro-portionate response was observed to the mutational load of each tumor.26
These data are exciting and, while preliminary, have resulted in the FDA granting a priority review to pembrolizumab for MSI-H metastatic CRC. Further, given the multiple ongoing studies, these data support the recommendation to more broadly screen patients for MSI, even in stage IV disease without a high suspicion for Lynch syndrome. An ongoing trial, KEYNOTE-164, is seeking to confirm these results, using a lower dose of pembrolizumab, 200 mg IV every 21 days (NCT02460198). This is a single-arm phase II study that enrolled 2 cohorts: those with refractory disease and those who have received at least 1 line of therapy. KEYNOTE-177 is an additional important study that is enrolling untreated patients with MSI-H or dMMR metastatic CRC (NCT02563002). In a 1:1 fashion, with a crossover design, this phase III study is assigning patients to standard of care chemotherapy or single-agent pembrolizumab. The primary endpoint is PFS, with secondary endpoints of overall response rate (ORR) and OS.
Durvalumab
Durvalumab (MEDI4736) is a humanized mAB directed against PD-L1. An ongoing investigation is evaluating durvalumab in a phase II study of locally advanced or metastatic MSI-H CRC with an elevated density of tumor-infiltrating lymphocytes (TILs) on biopsy (NCT02227667). This study is unique in that an assessment of tumor microenvironment is being utilized for inclusion.
CTLA-4 and PD-1 Combination Therapy
Because CTLA-4 inhibition (ipilimumab) and PD-1 inhibition (nivolumab) appear to offer improved response rates and PFS compared with either therapy alone in melanoma, there is hope that the combination might also offer broadened benefit in CRC. In CRC syngeneic mouse models, combined PD-1 and CTLA-4 inhibition achieves success where neither agent was capable of consistently doing so on its own.27The phase I/II CheckMate 142 study compared use of nivolumab monotherapy arm (3 mg/kg q2wk) with nivolumab (3 mg/kg q2wk) + ipilimumab (1 mg/kg x 4 doses) in patients with recurrent and metastatic MSI-H and MSS CRC (NCT02060188). Preliminary results were presented at the ASCO 2016 meeting. Seventy patients were treated in the nivolumab monotherapy arm, including 47 evaluable patients who had been on therapy with at least 12 weeks follow-up. Here, 12 (25.5%) patients achieved a partial response, with an additional 14 (30%) with stable disease, a disease control rate of 55.5%. The median duration of response had not yet been reached (range, 0 15.2 mos).
Thirty patients with MSI-H CRC were treated with combined nivolumab and ipilimumab. The results for the 27 evaluable patients with at least 12 weeks follow-up were presented, with 9 (33%) demonstrating a partial response and 14 (52%) with stable disease. This translates to an 85% disease control rate. The investigator assessed 6-month PFS appeared slightly better than nivolumab monotherapy: 66.6% versus 45.9%, respectively. Initially, the re- sponse rates and disease control appear superior to nivolumab monotherapy, but the small numbers and very limited follow-up time preclude any firm conclusions as to differential efficacy. Since responses with ipilimumab can be delayed, it will be highly interesting to see the mature follow-up data to establish whether there is a meaningful difference in activity which would support the further development and use of CTLA-4/PD-1 doublets despite concerns of increased toxicity.28Of 20 evaluable patients with MSS CRC who received combination therapy, a partial response was observed in 1 patient (5%). However, for the combination therapy group, PFS was poor, with the median standing at less than 3 months.
Combination Approaches
The story for MSI-H tumors appears promising. As we wait on further data, there is hope that the activity spectrum of checkpoint inhibitors can be expanded in CRC. Given the highly immunosuppressive tumor milieu, combination approaches or alternative agents would appear to be necessary for broader application. A selection of additional investigational approaches and initial results, when applicable, is described in the sections that follow.
Combination Approaches
Cobimetinib + Atezolizumab
Cobimetinib (Cotellic) is a selective inhibitor of mito-gen-activated protein kinase kinase (MEK). MEK is a dual specificity kinase, a key component of the RAS/ RAF/MEK/ERK pathway that is constitutively activated in a wide variety of tumors. Atezolizumab (Tecentriq) is an IgG1 mAb targeting PD-L1. In preclinical models, targeted inhibition of MEK leads to upregulation of MHC I on tumor cells, induces intratumoral T-cellin lnfiltration and synergizes with PD-1/L1 inhibition.29Results of a phase Ib clinical trial using the combination cobimetinib and atezolizumab in patients with advanced solid tumors were reported at the 2016 ASCO Annual Meeting (NCT01988896). Twenty-three patients enrolled had metastatic CRC (mCRC), 20 of whom compromised a KRAS-mutant CRC expansion cohort. Thirty percent of the patients were MSS or MSI-low (MSI-L), while 70% had unknown MSI status. Of great interest, 4 (17%) patients demonstrated objective response. Three of four were pMMR (MSS equivalent) and for 1 patient the status remains unknown. Six (26%) patients were on therapy for >12 months. Although median PFS was 2.3 months, 6-month OS was 72%. This compares favorably with regorafenib (Stivarga) and TAS-102, where the median OS stands at 6.4 and 7.1 months, respectively.30
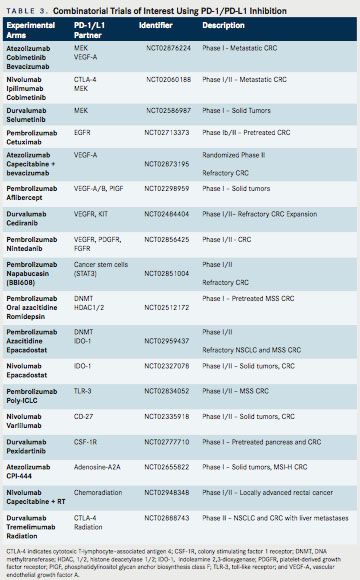
Of note, MEK inhibition alone has not previously demonstrated significant clinical activity in CRC, suggesting that MEK inhibition may be able to prime an immune response. This opens the door to related priming strategies. On the heels of these encouraging results, a phase III study has been launched, which is randomizing patients with refractory metastatic CRC to 1 of 3 arms: atezolizumab + cobimetinib, atezolizumab monotherapy, or regorafenib (NCT02788279). In addition, an ongoing phase I study is evaluating the efficacy of the VEGF-TRAP, anflibercept, combined with pembrolizumab, in patients with recurrent or mCRC. Multiple additional trials are underway examining combinations of targeted therapy with PD-1/PD-L1 inhibition (Table 3).
Chemotherapy + Immune Checkpoint Therapy
Chemotherapy is capable of immunomodulatory effects on its own. There are preclinical data to support 5-FU and gemcitabine as being toxic to suppressive myeloid-derived suppressor cells (MDSCs), with FOLFOX decreasing levels of peripherally circulating MDSCs in patients with CRC.31,32Drugs that target VEGF may have beneficial effects by reducing regulatory T cells.33However, scant clinical data have been presented on combined chemotherapeutics and checkpoint inhibitors across all cancers, and particularly in CRC. Thus, forgetting toxicity concerns for a moment, it remains uncertain clinically whether there will be a beneficial or detrimental impact of combination with chemotherapy.
Chemotherapy + Atezolizumab
Initial safety data from a multiarm study of atezolizumab were presented at the 2015 GI Cancers Symposium meeting (NCT01633970). In arm A, 14 patients with refractory CRC were treated with bevacizumab and atezolizumab. One (7%) partial response was seen, with 9 (64%) patients achieving stable disease, which lasted at least 24 weeks in 2 patients. In arm B, 30 patients (70% chemotherapy naïve) were treated with FOLFOX plus bevacizumab and atezolizumab. In this arm, of the 23 first-line patients, a 48% response rate was achieved with an 87% disease control rate (response or stable disease). Immune activation was demonstrated in the tumor biopsies and peripheral blood.34However, at this interim analysis, it was not clear whether or not there is an additional benefit conferred by adding PD-L1 blockade to standard therapy; the outcomes looked strikingly similar to those achieved via FOLFOX and bevacizumab alone. Similarly, it cannot be determined with certainty whether or not the observed immune activation differs from that which would be seen with use of chemotherapy alone. Of course, given the lack of single-agent antiPD-1 activity in patients with unselected CRC, such combinations might not improve response rates, but they may be capable of deepening or prolonging responses. Therefore, further follow-up analyses, including data on PFS and OS would be useful.
Additional studies are evaluating PD-1/PD-L1 inhibition and chemotherapy combinations in CRC. Pembrolizumab is being tested in combination with mFOLFOX6 in 2 ongoing clinical studies, one specifically for CRC (NCT02375672, NCT02268825).
Atezolizumab is being evaluated in a biomarker-driven multi-arm trial of various maintenance strategies in treatment-naïve metastatic CRC outside of the United States (NCT02291289). One arm will evaluate the combination of 5-FU, bevacizumab, and PD-L1 inhibition after initial induction therapy with mFOLFOX6 and bevacizumab. In refractory disease,the BACCI study will seek to evaluate the impact of adding atezolizumab to capecitabine and bevacizumab (NCT02873195). and other metastatic solid tumors (NCT02298959).
A study of particular importance will assess the benefit of adding PD-L1 inhibition, via atezolizumab, to adjuvant FOLFOX for patients with stage 3 MSI-H CRC (NCT02912559). This study is anticipated to open within the next 6 months through the national cooperative group system (Alliance). In this study, atezolizumab will be administered concurrently with FOLFOX and continued for a 1-year total course of therapy. The primary end point will be disease-free survival and will aim to accrue 720 patients. The adjuvant setting makes this unique compared with ongoing trials.
Radiotherapy and Immune Checkpoint Therapy
Radiation-induced cell death is capable of priming an antitumor immune response. Alterations in the tumor microenvironmentsuch as enhanced expression of death receptors, MHC class I molecules, costimulatory molecules, and release of various chemokines—can make tumor cells more susceptible to T-cell response. Investigators also believe that new antigens may be expressed or released in this process. In turn, this might allow for activated circulating immune cells to target malignant cells at sites distant from radiotherapy (RT)—the so-called abscopal effect.35Studies combining RT are ongoing across malignancies, including CRC. One completed study has evaluated the PD-1 inhibitor, AMP224, combined with liver directed stereotactic body radiation therapy (SBRT) in patients with metastatic CRC (NCT02298946). Although safe and feasible, no responses were observed at the time of reporting.36Another study of interest is evaluating the effects of radiofrequency ablation plus pembrolizumab versus external beam RT plus pembrolizumab in patients with mCRC (NCT02437071). Interim results were reported at the ASCO 2016 Annual Meeting. With 22 patients having received various doses of short course RT, 1 (4.5%) response has been reported in the first arm. No responses have been observed with utilization of cryo- or microwave ablation. Additional trials will study the role of long-course chemoradiation in locally advanced rectal cancer (NCT02948348, NCT02586610), while others will evaluate combined CTLA-4/PD-1 inhibition with varying doses of RT (NCT02888743). High levels of synergy have not been observed to date. These ongoing trials will help to determine whether this is an avenue worthy of ongoing pursuit.
Conclusions
Immunotherapy appears poised to make a major impact in the future management of CRC. Recently updated National Comprehensive Cancer Network guidelines highlight the importance of routinely evaluating MSI, recommending assessment of MSI (via PCR) or MMR (via immunohistochemistry) in all patients with diagnosed CRC. This is as essential as routine extended RAS testing is for patients with metastatic disease. To date, the MSI-H population represents the group of patients where PD-1 inhibition may prove effective. Initial data with CTLA-4 and PD-1 combinations suggest potential for additional benefit, although data remain immature and toxicity will remain a concern.
On the other hand, for MSS CRC there is no role for PD-1/L1 inhibition monotherapy. Combination therapy with PD-1 and CTLA-4 inhibition has suggested only limited activity to date for MSS CRC, although full data have yet to be presented. Predictive biomarkers are otherwise limited. PD-L1 expression alone does not appear to be useful, although tumor mutational burden may be useful someday. Combinatorial therapies likely hold the key to the future. Combined MEK and PD-L1 inhibition, via cobimetinib and atezolizumab, are demonstrating exciting preliminary signs of clinical activity. Confirmatory studies are ongoing, as are multiple additional combinatorial strategies. Ongoing participation of patients in clinical trials remains critical to accelerating progress in the treatment of CRC.
References:
- Amarnath S, Mangus CW, Wang JC, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3(111):111ra120. doi: 10.1126/ scitranslmed.3003130.
- Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015-3029. doi: 10.1084/jem.20090847.
- Schreiber RD, Old LJ, Smyth MJ.Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565-1570. doi: 10.1126/science.1203486.
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568-571. doi: 10.1038/nature13954.
- JohnsenAK,TempletonDJ,SyM,HardingCV.De ciencyoftransporterfor antigen presentation (TAP) in tumor cells allows evasion of immune surveillance and increases tumorigenesis. J Immunol. 1999;163(8):4224-4231.
- Spranger S, Gajewski TF. A new paradigm for tumor immune escape: beta- catenin-driven immune exclusion. J Immunother Cancer. 2015;3:43. doi: 10.1186/ s40425-015-0089-6.
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525- 532.
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361(25):2449-2460. doi: 10.1056/ NEJMra0804588.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5): 646-674. doi: 10.1016/j.cell.2011.02.013.
- Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101(4):256-266. doi: 10.1093/jnci/djn48.
- Rothwell PM, Wilson M, Elwin CE, et al. Long-term e ect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of ve randomised trials. Lancet. 2010;376(9754):1741-1750. doi: 10.1016/S0140-6736(10)61543-7.
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960-1964.
- Mei Z, Liu Y, Liu C, et al. Tumour-in ltrating in ammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110(6):1595-1605. doi: 10.1038/bjc.2014.46.
- Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20(7):1891- 1899. doi: 10.1158/1078-0432.CCR-13-2830.
- Koopman M, Kortman GA, Mekenkamp L, et al. De cient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100 (2):266-273. doi: 10.1038/sj.bjc.6604867.
- Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073-2087 e3. doi: 10.1053/j.gastro.2009.12.064.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609-618.
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of e cacy of uorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219-3226. doi: 10.1200/JCO.2009.27.1825.
- Tougeron D, Mouillet G, Trouilloud I, et al. E cacy of adjuvant chemotherapy in colon cancer with microsatellite instability: a large multicenter AGEO study. J Natl Cancer Inst. 2016;108 (7). pii: djv438. doi: 10.1093/jnci/djv438.
- Maby P, Tougeron D, Hamieh M, et al. Correlation between density of CD8+ T-cell in ltrate in microsatellite unstable colorectal cancers and frameshift mutations: a rationale for personalized immunotherapy. Cancer Res. 2015;75(17):3446-3455. doi:10.1158/0008-5472.CAN-14-3051.
- LlosaNJ,CruiseM,TamA,etal.Thevigorousimmunemicroenvironmentof microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43-51. doi: 10.1158/2159-8290.
- ChungKY,GoreI,FongL,etal.PhaseIIstudyoftheanti-cytotoxicT-lymphocyte- associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28(21):3485-3490. doi: 10.1200/JCO.2010.28.3994.
- TopalianSL,HodiFS,BrahmerJR,etal.Safety,activity,andimmunecorrelates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690.
- LipsonEJ,SharfmanWH,DrakeCG,etal.Durablecancerregressiono - treatment and e ective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(2):462-468. doi: 10.1158/1078-0432.CCR-12-2625.
- LeDT,UramJN,WangH,etal.PD-1Blockadeintumorswithmismatch- repair de ciency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/ NEJMoa1500596.
- LeDTUJ,WangH,BartlettBR,etal.PD-1Blockadeintumorswithmismatch- repair de ciency. J Clin Oncol. 2016;34(suppl; abstr 103).
- DuraiswamyJ,KaluzaKM,FreemanGJ,CoukosG.DualblockadeofPD-1and CTLA-4 combined with tumor vaccine e ectively restores T-cell rejection function in tumors. Cancer Res. 2013;73(12):3591-3603. doi: 10.1158/0008- 5472.CAN-12-4100.
- OvermanMJKS,McDermottRS,etal.Nivolumab±ipilimumabintreatment(tx)of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. J Clin Oncol 2016;34(suppl; abstr 3501).
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291-3310.
- Bendell JC KT, Goh BC, Wallin J, Oh D-Y, et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). J Clin Oncol. 2016;34(suppl; abstr 3502).
- Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor- associated myeloid-derived suppressor cells resulting in enhanced T cell- dependent antitumor immunity. Cancer Res. 2010;70(8):3052-3061. doi: 10.1158/0008-5472.CAN-09-3690.
- Kanterman J, Sade-Feldman M, Biton M, et al. Adverse immunoregulatory e ects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014;74(21):6022-6035. doi: 10.1158/0008-5472.CAN-14-0657.
- Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73(2): 539-549. doi: 10.1158/0008-5472.CAN-12-2325.
- Bendell JC PJ LC, et al. Safety and e cacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol. 2015;33(suppl 3: abstract 704).
- Formenti SC, Demaria S Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256-265. doi: 10.1093/jnci/djs629.
- Du y AG M-RO, Pratt D, et al. A pilot study of AMP-224, a PD-L2 Fc fusion protein, in combination with stereotactic body radiation therapy (SBRT) in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(suppl 4S; abstr 560).
- BrahmerJR,TykodiSS,ChowLQ,etal.Safetyandactivityofanti-PD-L1antibody inpatientswithadvancedcancer.NEnglJMed.2012;366(26):2455-2465.doi: 10.1056/NEJMoa1200694.
