First-line Niraparib Improves PFS in Ovarian Cancer
An individualized starting dose of niraparib, determined by weight and platelet count, continued to demonstrate a clinical meaningful improvement in progression-free survival in newly diagnosed ovarian cancer in the first-line maintenance setting, regardless of biomarker status,
Ning Li, MD
An individualized starting dose of niraparib (Zejula), determined by weight and platelet count, continued to demonstrate a clinical meaningful improvement in progression-free survival (PFS) in newly diagnosed ovarian cancer in the first-line maintenance setting, regardless of biomarker status, according to data from the phase 3 PRIME study (NCT03709316) presented during the 2022 SGO Annual Meeting on Women’s Cancer.
The median PFS in the intent-to-treat (ITT) population was 24.8 months (HR, 0.45; 95% CI, 19.2–not estimated [NE]) in the nirapa- rib arm compared with 8.3 months (95% CI, 7.3-11.1) in the placebo group (HR, 0.45; 95% CI, 0.34-0.60; P < .001). The homologous recombination deficiency (HRD) subgroup had a median PFS that was not reached (NR; 95% CI, 22.3-NE) in the niraparib arm vs 11.0 months (95% CI, 8.3-13.8) in the placebo group (HR, 0.48; 95% CI, 0.34-0.68; P <.001).
Patients with BRCA mutations had a median PFS that was NR (95% CI, 22.3-NE) vs 10.8 months (95% CI, 8.3-19.3) in the niraparib and placebo cohorts, respectively (HR, 0.40; 95% CI, 0.23-0.68; P < .001). Those with non–germline BRCA (non-gBRCA) mutations had a median PFS of 19.3 months (95% CI, 13.8-NE) vs. 8.3 months (95% CI, 5.6-11.2) in the niraparib and placebo groups, respectively (HR, 0.48; 95% CI, 0.34-0.67; P < .001).
In the non-gBRCA mutation subgroups, those who were HRD had a median PFS of 24.8 months (95% CI, 14.0-NE) vs 11.1 months (95% CI, 8.3-13.8) in the niraparib and placebo groups, respectively (HR, 0.58; 95% CI, 0.36-0.93; P = .022).
For those with non–gBRCA mutations who were homologous recombination proficient, the median PFS was 14.0 months (95% CI, 11.9-NE) vs 5.5 months (95% CI, 2.9-7.3) in the niraparib and placebo groups, respectively (HR, 0.41; 95% CI, 0.25-0.65; P < .001).
“PRIME data continue to support niraparib monotherapy after first-line platinum-based chemotherapy regardless of biomarker status,” Ning Li, MD, Cancer Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, said during the presentation.
A total of 384 patients were randomized 2:1 and received either niraparib (n = 255) or placebo (n = 129) for 36 months or until disease progression, death, or unacceptable toxicity. Patients were eligible for treatment if they had FIGO stage III or IV ovarian cancer, a high-grade or serous endometrioid tumor, previous primary or interval cytoprotective surgery despite postoperative residual disease status, and a complete (CR) or partial response (PR) to first-line platinum-based chemotherapy.
Stratification factors included germline BRCA mutational status, tumor HRD status, previous neoadjuvant chemotherapy, and response to first-line platinum chemotherapy. The primary end point was PFS via blinded independent central review in the ITT population. Secondary end points were OS, time to first subsequent anti- cancer therapy in the ITT subgroup, PFS and OS in the HRD subgroup, and safety.
At data cut-off, 102 patients in the niraparib group and 29 in the placebo were still receiving treatment. The median follow-up was 27.5 months.
Patient characteristics included a median age of 53.0 years in the niraparib group vs 54.0 years in the placebo, and a median weight of 59.0 kg in the niraparib group and 57.0 kg in the placebo group. A total of 71.4% of patients in the niraparib group had FIGO stage III disease vs 72.9% in the placebo and 28.6% vs 27.1% of patients in both respective groups had stage IV disease.
A total of 83.1% and 79.8% experienced a CR to previous platinum-based chemotherapy in the niraparib and chemotherapy groups, respectively. Prior treatment with chemotherapy resulted in a PR in 16.9% vs 20.2% of patients in each respective group. A total of 33.3% of patients in the niraparib group and 31.0% in the placebo group had a germline BRCA mutation.
At 24 months, 52.6% in the niraparib group and 30.4% in the placebo group did not have progressive disease or death. Those in the HRD subgroup without progressive disease or death included 57.4% in the niraparib group and 33.9% in the placebo group.
OS data were immature in the ITT population (HR, 0.63; 95% CI, 0.38-1.03; P = .061). The median time to first subsequent anti-cancer therapy was 29.2 months (95% CI, 22.4–NE) in the niraparib group and 11.9 months (95% CI, 8.8-14.8) in the placebo group.
"While overall survival [OS] data are still immature, there is a trend in favor of niraparib at this data cut-off,” Li said.
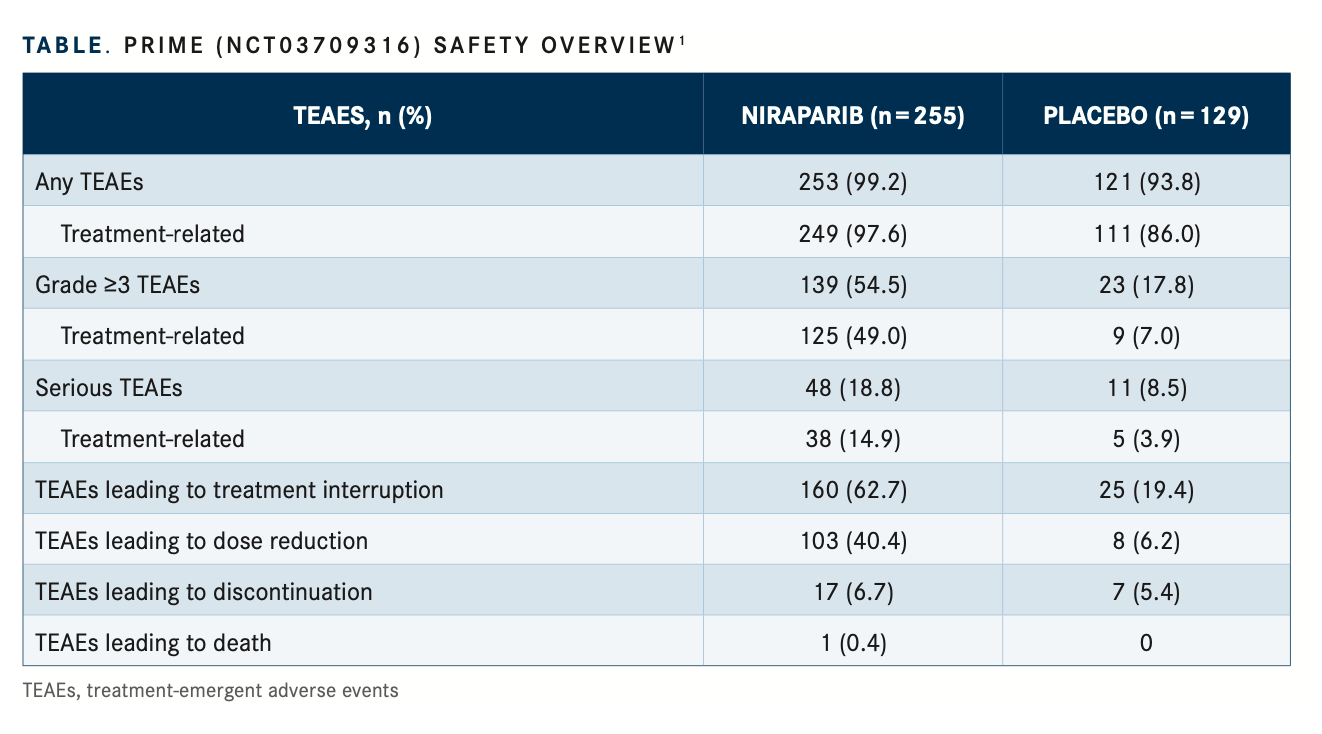
Grade 3 or higher treatment-emergent adverse events (TEAEs) were observed in 54.5% vs 17.8% of patients in the niraparib and placebo groups, respectively. Grade 3/4 treatment-related AEs were observed in 49.0% vs 7.0% in the niraparib and placebo groups, respectively. Serious TEAEs were observed in 18.8% vs 8.5% of patients in the niraparib and placebo groups, respectively, of which 14.9% vs 3.9% treatment related (TABLE1).
In the niraparib and placebo cohorts, TEAEs relating to treatment interruption were observed in 62.7% vs 19.4%, dose reductions in 40.4% vs 6.2%, discontinuation in 6.7% vs 5.4%, and death in 0.4% vs 0% of patients, respectively. Common grade 3 or higher TEAEs observed in the niraparib and placebo cohorts, respectively, were decreased neutrophil count (17.3% vs 1.6%), decreased white blood cell count (6.7% vs 0.8%), anemia (18.0% vs 1.6%), decreased platelet count (14.1% vs 0.8%), and increased glutamyltransferase (5.1% vs 17.1%).
REFERENCE
Li N, Zhu J, Yin R, et al. Efficacy and safety of niraparib as maintenance treatment in patients with newly diagnosed advanced ovarian cancer using an individualized starting dose (PRIME Study): a randomized, double-blind, placebo-controlled, phase 3 trial. Presented at: 2022 SGO Annual Meeting on Women’s Cancer; March 18-21, 2022; Phoenix, AZ.
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More