EZH2 Targetability Demonstrated Across Cancer Types
The disruption of chromatin modulation has been shown to be an important step in the development of certain cancers. A variety of cancer types exhibit chromatin-modifying mutations, which often correlate with cell fate decisions. Specifically, mutations in EZH2 (enhancer of zeste homolog 2) have been frequently observed in cancer, and small molecule inhibitors have been developed against the enzymatic activity of EZH2, with evidence of clinical activity in early-phase trials.
EZH2(enhancer of zeste homolog 2) have been frequently observed in cancer, and small molecule inhibitors have been developed against the enzymatic activity ofEZH2, with evidence of clinical activity in early-phase trials.
“Some specific cancers are based on ‘gain-of -function’ mutations inEZH2,” noted Thomas M. Kristie, PhD, chief of the Molecular Genetics Section in the Laboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases in Bethesda, Maryland, in a recent news release.1“Additionally, it has been proposed that in some cancers, these enzymes repress anti-oncogenes, and treatment with EZH2/1 inhibitors might result in re-expression of these anti-oncogenes.”
TheEZH2gene encodes the protein EZH2, a histone methyltransferase and the core enzymatic subunit of polycomb repressive complex 2 (PRC2), which is involved in chromatin compaction and transcriptional silencing (FIGURE). EZH2 functions to methylate a lysine residue (K27) on the N-terminal region of histone H3. These activities are part of an emerging class of epigenetic modifiers attracting interest as anticancer targets.
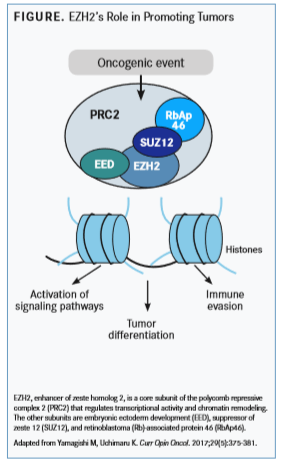
EZH2 overexpression has been associated with enhanced progression and advanced disease in cancers of the prostate, bladder, breast, and endometrium, as well as melanoma. High expression of EZH2, defined as greater than 10% in immunohistochemical analysis and up to 4-fold increased expression via real-time polymerase chain reaction testing, has been associated with increased disease aggressiveness.2EZH2 has also been implicated in tumor initiation and progression, migration, angiogenesis, stem cell self-renewal, and activated T-regulatory cell functioning.
During development, EZH2/PRC2 has been shown to silence genes associated with alternative fates at specific loci. As in normal stem cells, EZH2 is highly expressed in cancer stem cell populations and suppresses differentiation via repression of lineage-specific factors to maintain these populations. It has been hypothesized that EZH2 blocks differentiation, which facilitates cell transformation.
The retinoblastoma-E2F pathway, which regulates the cell cycle, has been demonstrated to be upstream of EZH2, which is required for proliferative gene expression and E2F-driven proliferation. It is unknown whether any EZH2 targets are essential for EZH2-mediated transformation across all cell types or whether oncogenic changes are cell type-specific.
EZH2 may have a role as a transcriptional coactivator independent of PRC2, leading to direct modulation of transcription factor activity; however, these functions have not been fully elucidated.
Agents in development are highly specific to EZH2 in order to avoid interfering with nontumorigenic pathways. Inhibition of EZH2 methyltransferase activity without degrading the PRC2 complex is achieved through competition with the methyl-donor S-adenosyl methionine (SAM) for the EZH2 binding pocket; therefore, these agents are known as SAM-competitive inhibitors.
TAZEMETOSTAT
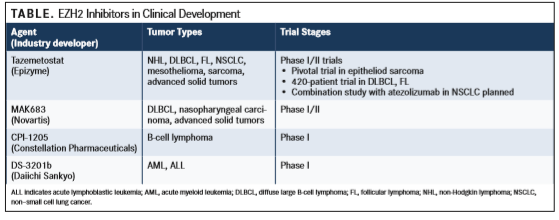
The small-molecule EZH2 inhibitor most advanced in its development is tazemetostat, which is orally administered (TABLE). Interim phase II tazemetostat safety data were presented at the 14th International Conference on Malignant Lymphoma in June 2017.3Tazemetostat was well tolerated and demonstrated clinical activity in early clinical trials of patients with genetically defined solid tumors and hematologic malignancies.
Tazemetostat was studied in an open-label multicenter study in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) or follicular lymphoma (FL) who received tazemetostat 800 mg twice daily. Grade 3 or higher treatment-emergent adverse events (AEs) were reported in 18% of patients. Any-grade AEs reported in more than 5% of patients included nausea (20%), thrombocytopenia (19%), cough (14%), diarrhea (11%), fatigue (12%), and asthenia (10%).
Among participants evaluable for efficacy on June 1, the objective response rate (ORR) was 29% among patients withEZH2-mutated DLBCL (5 of 17 patients) and 15% among those with wild-type disease (18 of 119). For patients with FL, the ORR was 92% for those with the mutation (12 of 13) and 26% for those with wild-type disease (14 of 54).
These findings demonstrate preliminary clinical activity and a favorable safety profile in patients receiving tazemetostat, with enhanced benefit in patients with tumors that have activatingEZH2mutations. Because it has been previously reported that clinical response onset may be delayed or evolve from stable disease to partial response (PR) or from PR to complete response (CR), patient follow-up is ongoing.
Franck Morschhauser, MD, PhD, with the Centre Hospitalier Régional Universitaire de Lille in France and lead investigator of the study, stated that he was “encouraged by the level of activity in DLBCL patients withEZH2mutations, especially in light of the bleak prognosis associated with advanced disease. Tazemetostat has demonstrated a uniquely tolerable safety profile, and I look forward to further exploring its full benefit in patients with relapsed or refractory FL and DLBCL as the data mature.”
Tazemetostat has also shown antitumor activity in preclinical malignant rhabdoid tumor (MRT) models that were negative for the INI1 protein and in phase I trials of MRT and epithelioid sarcoma.4
In a phase II multicenter, open-label study, 31 adult patients with epithelioid sarcoma whose tumors indicated loss of INI1, a hallmark of the malignancy, were enrolled and treated with tazemetostat 800 mg twice daily. The primary endpoint was disease control rate, which was defined as stable disease lasting at least 32 weeks or an objective response of any duration. Stage II success required disease control rate in at least 5 treated patients.
These criteria were surpassed, with confirmed PRs in 4 patients and stable disease in 2 patients at time of publication. Thirteen patients remained on treatment and updated results will be presented. The most frequently reported any-grade AEs included fatigue in 34% of patients and dyspnea and nausea in 27% each, demonstrating that tazemetostat was well tolerated in this patient population. Based on these findings, enrollment has been expanded to 60 patients with epithelioid sarcoma.
“Epithelioid sarcoma is a difficult cancer for sarcoma oncologists like me to treat due to there being few available therapeutic options, which are associated with limited benefit and challenging side effects for patients,” said Mrinal M. Gounder, MD, attending physician at Memorial Sloan Kettering Cancer Center in New York City and lead investigator in the phase II clinical trial, in a press release. “INI1 loss is a defining feature of epithelioid sarcoma, and the mechanism of tazemetostat makes this a compelling agent. These data show encouraging activity of tazemetostat as characterized by objective responses, duration of responses, and prolonged disease stabilization.”
Tazemetostat is being tested in combination with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) as a first-line treatment for newly diagnosed high-risk, elderly patients (60-80 years) with DLBCL in the phase I/II Epi-RCHOP study (NCT02889523). In phase I, the primary outcome will be dose-limiting toxicities and secondary outcomes will be serum concentration of CHOP components with and without tazemetostat and serum concentration of tazemetostat in the presence of CHOP. In the phase II study, the primary outcome will be CR rate, with secondary endpoints of overall response rate, progression-free survival (PFS), and serum concentrations of CHOP components and tazemetostat.
The drug is also being evaluated in a phase I study as a combination therapy with atezolizumab (Tecentriq), an antiPD-L1 immunotherapy, and in a phase Ib study as a combination therapy with prednisolone, in patients with relapsed or refractory DLBCL (NCT02220842, NCT01897571).
Additionally, tazemetostat is among the first investigational therapies to be tested in the multicenter, phase II Pediatric MATCH trial, which is being sponsored by the National Cancer Institute. The trial is examining targeted agents in children with advanced metastatic/recurrent tumors that are refractory and haveEZH2,SMARCA4, orSMARCB1gene mutations. The primary endpoint will be ORR, with a secondary endpoint of PFS and additional evaluations of biomarker predictors of treatment response and change in tumor dynamics (NCT03213665, NCT03155620).
In April 2017, the FDA granted a Fast Track designation to tazemetostat for relapsed/refractory FL with or without anEZH2-activating mutation. The agency previously granted the drug Fast Track status in 2016 for patients with relapsed/refractory DLBCL withEZH2-activating mutations. In June 2017, the FDA approved an orphan drug designation to tazemetostat for soft tissue sarcoma.
OTHER EMERGING INHIBITORS
MAK683 is a compound that binds embryonic ectoderm development (EED) protein, which is the part of PRC2 and attaches to the DNA packaging protein. MAK683 functions by changing the overall shape of PRC2, leading to inactivation of the complex. In a murine model of B-cell lymphoma, MAK683 altered the expression of several hundred genes and induced tumor regression. Currently, MAK683 is under investigation in a phase I/II trial of patients with nasopharyngeal carcinoma, DLBCL, and other advanced solid tumors for which no effective treatments exist (NCT02900651).
CPI-1205 binds directly to the EZH2 catalytic pocket, partially overlapping with the SAM binding site. This compound reversibly inhibits PRC2 enzymatic activity and reduces global methylation levels. PRC2 target gene expression has been altered in lymphoma cell lines, with dose-and time-dependent cell killing also caused by CPI-1205. In DLBCL xenograft models, CPI-1205 caused complete regression. This compound was well tolerated in toxicology investigations. A phase I trial has been launched (NCT02395601).
DS-3201b simultaneously inhibits the function of EZH1 and EZH2, and has been shown to reverse inappropriate histone methylation in adult T-cell leukemia-lymphoma cells and to selectively eliminate these cells and HTLV-1infected immortalized cells in peripheral blood. A phase I trial is ongoing (NCT03110354).
GSK2816126 is a highly potent and selective inhibitor of mutant and wild-type EZH2 that has been shown to decrease histone H3 trimethylation to induce antiproliferative activity in cancer cell lines and release transcriptional repression of PRC2 target genes. In a phase I study of 30 patients with DLBCL, FL, or other non-Hodgkin lymphoma, GSK2816126 was well tolerated and no dose-limiting toxicities were reported.5Frequently reported drug-related AEs included fatigue, nausea, vomiting, and anemia. Of 22 evaluable patients, 7 had stable disease and 1 durable PR was confirmed. Despite these promising results, the study was later terminated due to a lack of clinical activity.
Further evaluation of these agents in combination with conventional chemotherapeutic agents in preclinical and clinical studies may elucidate a beneficial treatment approach using this class of epigenetic modifiers.
References
- Epigenetic drugs show promise as antivirals [news release]. Washington, DC; ASM Communications; August 15, 2017. www.asm.org/index.php/newsroom/ item/6748-epigenetic-drugs-show-promise-as-antivirals. Accessed September 6, 2017.
- Ramaglia M, D’Angelo V, Iannotta A, et al. High EZH2 expression is correlated to metastatic disease in pediatric soft tissue sarcomas.Cancer Cell Int.2016;16:59. doi: 10.1186/s12935-016-0338-x.
- Morschhauser F, Salles G, McKay P, et al. Interim report from a phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory B-cell non-Hodgkin lymphomas [ICML abstract 4].Hematol Oncol.2016;16:59. 2017;35(suppl S2):24-25. onlinelibrary.wiley.com/doi/10.1002/hon.2437_3/epdf.
- Gounder MM, Stacchiotti S, Schöffski P, et al. Phase 2 multicenter study of the EZH2 inhibitor tazemetostat in adults with INI1 negative epithelioid sarcoma (NCT02601950). [ASCO abstract 11058]J Clin Oncol.2017;35 (suppl). meetinglibrary.asco. org/record/153327/abstract
- Yap TA, Winter JN, Leonard JP, et al. A phase I study of GSK2816126, an enhancer of zeste homolog 2 (EZH2) inhibitor, in patients (pts) with relapsed/ refractory diffuse large B-cell lymphoma (DLBCL), other non-Hodgkin lymphomas (NHL), transformed follicular lymphoma (tFL), solid tumors and multiple myeloma (MM). Presented at: 2016 American Society of Hematology Annual Meeting; December 3-6, 2016; San Diego, CA. Abstract 4203. ash.confex. com/ash/2016/ webprogram/Paper93812.html.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More