Exploring Adjuvant Therapies in Renal Cell Carcinoma
Although numerous therapeutics are available for the management of metastatic renal cell carcinoma in the first-line setting, there are few options and little guidance on the approach to adjuvant therapy.
Wenxin (Vincent) Xu, MD

Most patients renal cell carcinoma (RCC) initially present with localized disease that is curable with surgical intervention (complete nephrectomy).1,2 Unfortunately, 20% to 40% of those with localized primary tumors will develop metastatic disease, and approximately 1 in 4 patients with RCC have locally advanced or metastatic disease at initial presentation.3 Although numerous therapeutics are available for the management of metastatic RCC in the first-line setting, such as VEGFR inhibitors, mTOR inhibitors, and immune checkpoint inhibitors (ICIs), there are few options and little guidance on the approach to adjuvant therapy.4

Wenxin (Vincent) Xu, MD, medical oncologist and instructor, Dana-Farber Cancer Institute, Harvard Medical School, Boston, Massachusetts, and Rana R. McKay, MD, medical oncologist, associate professor of medicine, University of California, San Diego, recently discussed the background of adjuvant therapy for RCC, the recent KEYNOTE-564 (NCT03142334) study results and approval of pembrolizumab (Keytruda), and upcoming trials during interviews with Targeted Therapies in OncologyTM.
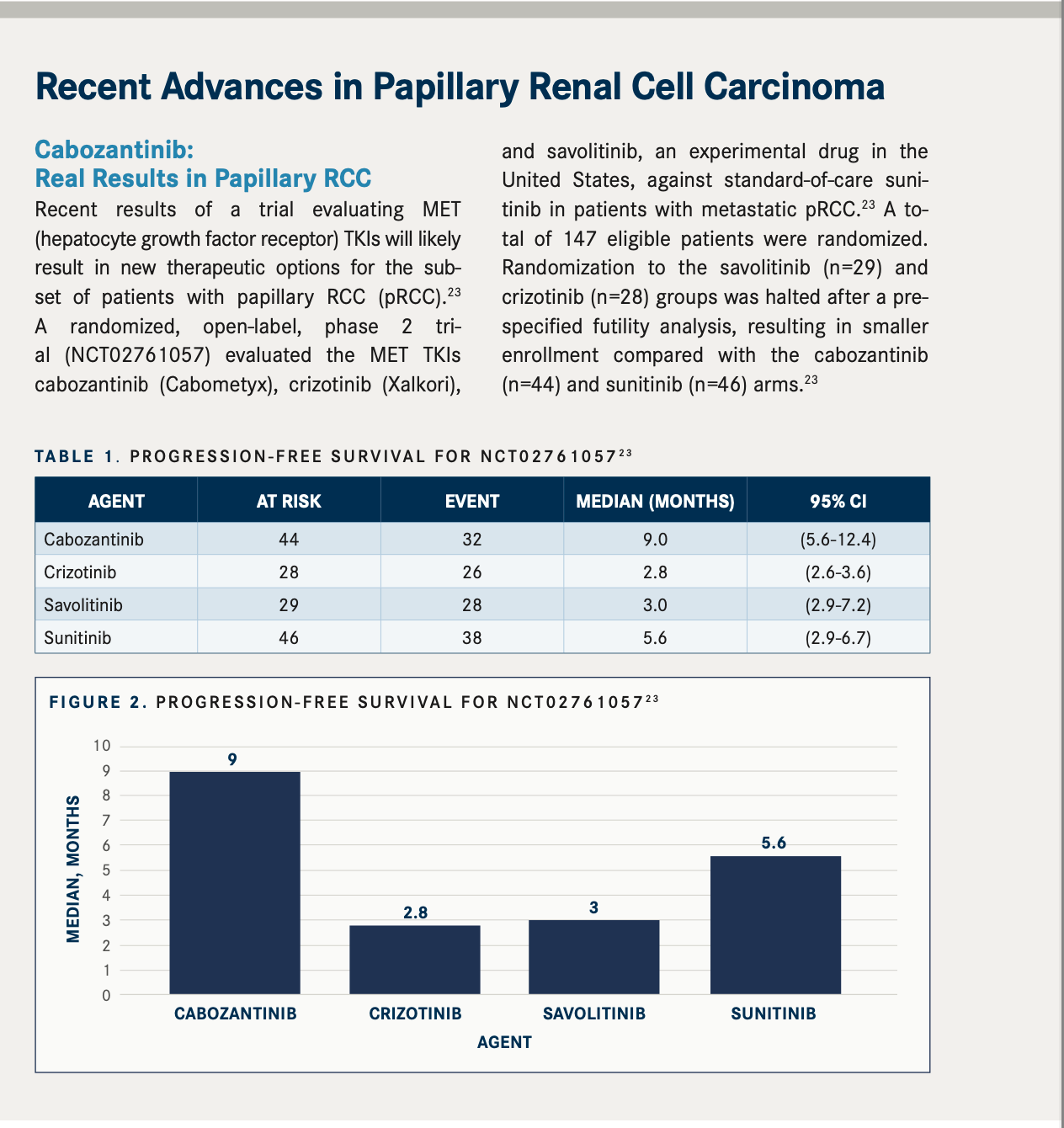
Sunitinib: Approved, but Limited Use
The first agents to be thoroughly evaluated in the adjuvant setting for RCC were tyrosine kinase inhibitors (TKIs), including sunitinib (Sutent; S-TRAC [NCT00375674], ASSURE [NCT00326898]), sorafenib (Nexavar; SORCE [NCT00492258]), axitinib (Inlyta; ATLAS [NCT01599754]), and pazopanib (Votrient; PROTECT [NCT01235962]).5 Of the TKIs assessed in these 5 trials, only 1 was subsequently approved as adjuvant treatment following nephrectomy for adult patients with high risk of recurrent RCC: sunitinib, a multitarget TKI that inhibits VEGF.
Approval was based on results from the S-TRAC trial, which included 615 patients with locoregional, high-risk clear cell RCC who received sunitinib or placebo on a 4-weeks-on, 2-weeks-off schedule for 1 year.6,7 This trial produced a median disease-free survival (DFS) of 6.8 years (95% CI, 5.8-not reached) for patients receiving sunitinib vs 5.6 years (95% CI, 3.8-6.6) for patients receiving placebo (HR, 0.76; 95% CI, 0.59-0.98; P =.03). The sunitinib group experienced a greater percentage of grade 3 (48.4%) and 4 (12.1%) adverse events (AEs) compared with the placebo group (grade 3, 15.8%; grade 4, 3.6%), but the incidence of serious AEs was similar, at 21.9% for sunitinib and 17.1% for placebo.6 Based on these data, the FDA approved sunitinib for the adjuvant treatment of adult patients at high risk of recurrent RCC following nephrectomy on November 16, 2017.7
However, the use of sunitinib has been limited, given the negative results from the phase 3, double-blind, placebo-controlled ASSURE trial.3,8,9 Eligible patients had completely resected, nonmetastatic, high-grade, stage T1b or greater RCC. Of the 647 patients randomly assigned to sunitinib, 438 received the full starting dose of 50 mg, and 193 (44%) of them discontinued the drug over toxicity issues. Because of this high rate of discontinuation, the dose was reduced to 37.5 mg for the remaining 191 patients, which resulted in a lower rate of withdrawal (34%).8 There were 4 treatment-related deaths in the sunitinib group; causes were neurological sequelae, sequelae of gastric perforation, pulmonary embolus, and disease progression. The investigators concluded that the study findings constitute a strong rationale against the use of adjuvant sunitinib in this setting.8
Sunitinib was [approved by the FDA] because there wasn’t anything else at the time,” said Xu. However, he admits sunitinib use in cancer treatment centers has been limited since its approval. Recently, a systematic review and meta-analysis of the 5 TKI trials previously discussed revealed that when the studies’ data were pooled, TKI therapy produced a significant DFS advantage vs placebo (pooled HR, 0.88; 95% CI, 0.81-0.96; P =.004), although this did not translate into a significant overall survival (OS) benefit (pooled HR, 0.93; 95% CI, 0.83-1.04; P =.23).5 In light of the finding that TKIs led to greater toxicity than placebo, the investigators concluded that the risk-benefit ratio is not supportive of TKI therapy in the adjuvant setting for most patients with RCC.5
Xu also mentioned that the rationale behind anti-angiogenic therapy has always been a source of debate. “VEGFR TKIs fundamentally work by limiting vascular supply to tumors, and micrometastatic tumor cells aren’t very constrained by their vascular supply until they reach a certain size. The concept of limiting this with antiangiogenic therapy was always controversial. Biologically, it is more plausible that the immune system can be more effective at removing these tumors.”
Trials in Adjuvant Therapy for RCC
Immuno-oncology is the current primary focus across oncology, and first-line regimen guidelines published by the National Comprehensive Cancer Network already include the use of ICI combinations for patients with RCC.10
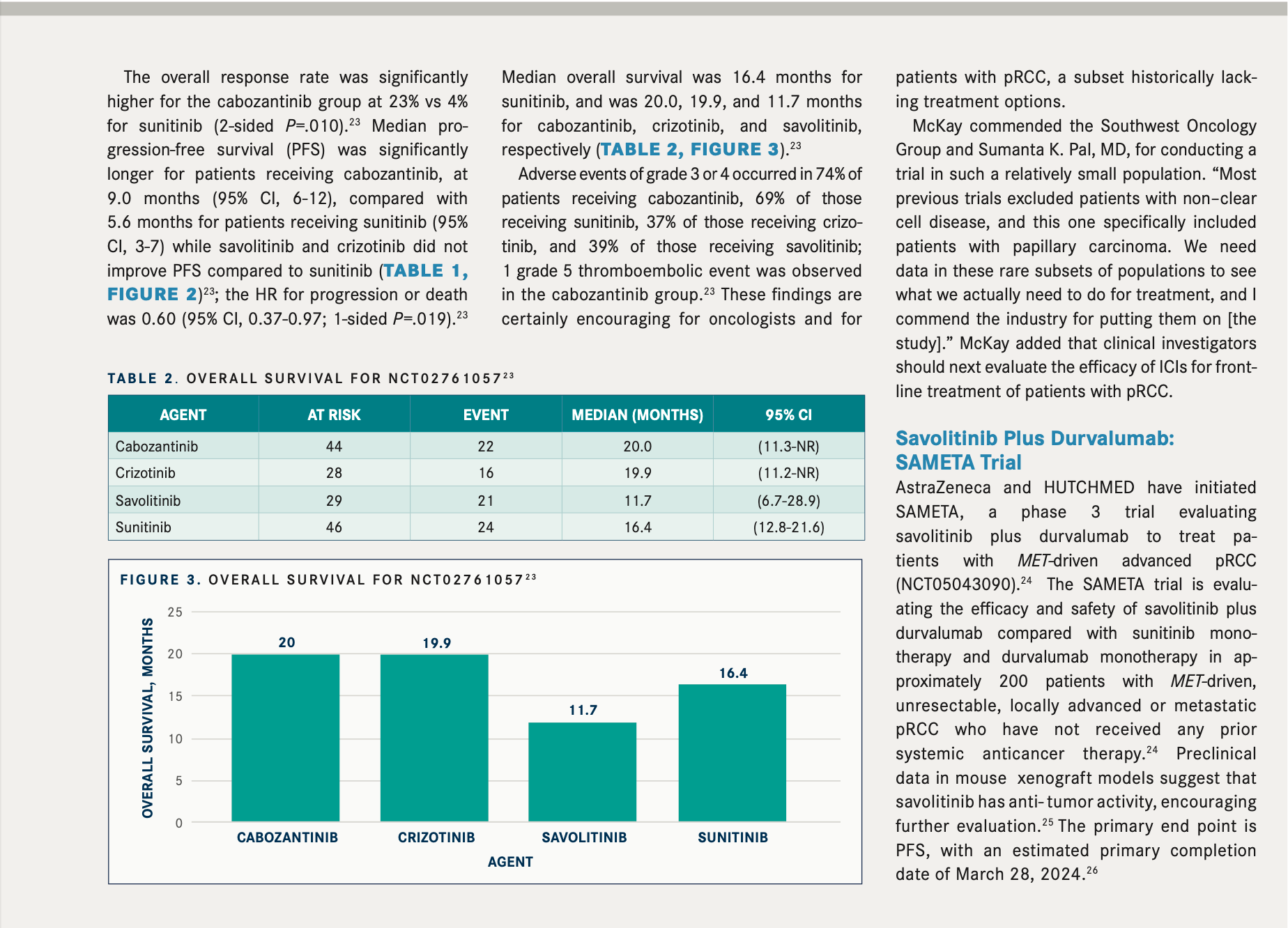
Pembrolizumab: KEYNOTE-564 and FDA Approval
Immuno-oncology is also the most promising avenue for adjuvant treatment of RCC, given recent results from a prespecified interim analysis of the KEYNOTE-564 trial (NCT03142334). Trial results led to the approval of the anti–PD-1 monoclonal antibody pembrolizumab for adults with RCC at increased risk of recurrence following nephrectomy or following nephrectomy and resection of metastatic lesions.
FDA approval was announced November 17, 2021, followed by the European Commission on January 27, 2022.11,12 This news represents a revolution in the adjuvant treatment of RCC as it provides an alternative to the only previously available option, sunitinib.7
The double-blind, randomized, phase 3 KEYNOTE-564 trial evaluated pembrolizumab (n = 496) vs placebo (n = 498) in patients with clear cell RCC at high risk of recurrence after nephrectomy, with or without removal of metastases.9
During the 2022 Genitourinary Cancers Symposium, updated efficacy and safety analysis with 30.1 months of follow-up were presented.13 DFS was maintained in patients receiving pembrolizumab (HR, 0.63; 95% CI, 0.50-0.80; nominal P < .0001). Three subgroups were analyzed: intermediate-high risk, high-risk, and M1 no evidence of disease (NED) following surgery. Findings across the subgroups were HR, 0.68; 95% CI, 0.52-0.89; HR, 0.60; 95% CI, 0.33-1.10; and HR, 0.28; 95% CI, 0.12-0.66, respectively.13
AEs of grade 3 or higher were observed in 32.4% of patients receiving pembrolizumab and 17.7% of those receiving placebo.9 Serious AEs occurred in 20% of patients in the pembrolizumab arm, pembrolizumab was discontinued in 21% of patients because of toxicity, and dose interruptions due to AEs occurred in 26% of patients.14
Although it will take some time to determine the impact on OS, McKay sees these results as potentially revolutionary for the field.
“These results have moved us forward into a new era for considering adjuvant therapy in cancer,” McKay said. “This is the beginning of the adjuvant immunotherapy era across solid tumor malignancies. I think it’s going to change how we view adjuvant therapy, referral patterns, and enhance the multidisciplinary nature of adjuvant therapy and treating RCC.”
Atezolizumab: IMmotion010 Trial
Another monoclonal antibody under investigation for adjuvant therapy in RCC is the anti– PD-L1 antibody atezolizumab (Tecentriq). The start of the phase 3, randomized, multicenter, double-blind IMmotion010 trial (NCT03024996) was announced in 2017 at the American Society of Clinical Oncology Annual Meeting.15 The aim of this study is to evaluate the efficacy and safety of atezolizumab in patients with RCC at high risk of recurrence after resection. The primary end point is DFS, with OS as a secondary end point. No data are available because analysis will not be conducted until approximately 65% of patients in both study populations have died.15
According to the latest update on ClinicalTrials.gov, 778 patients were enrolled when accrual was complete, and the estimated primary completion date is May 3, 2022.16 Oncologists in the genitourinary field are eagerly awaiting the results of this study, given that atezolizumab has had mixed outcomes in patients with RCC.17,18
“IMmotion010 with atezolizumab has a similar design to KEYNOTE-564,” McKay noted, “but atezolizumab has had a tough time in RCC, and we don’t use it to treat advanced disease. We’ll have to see if the data are positive for disease in the localized setting post surgery.”
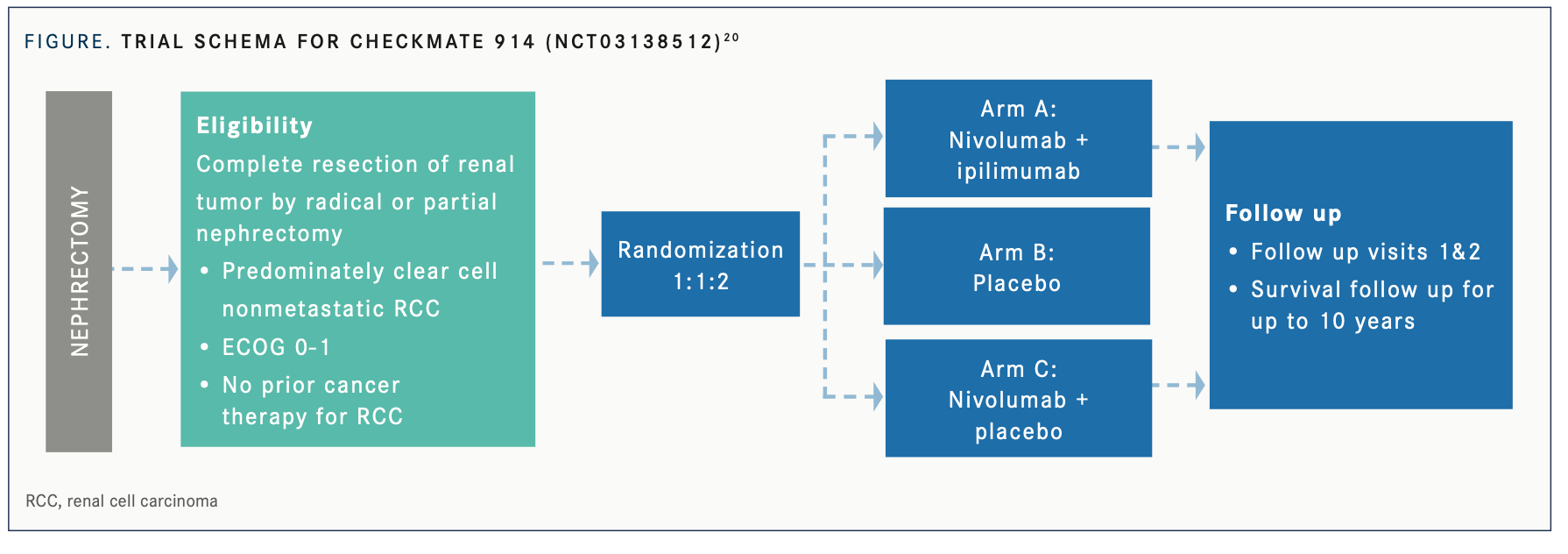
Nivolumab Plus Ipilimumab: CheckMate 914 Trial
The combination of nivolumab (Opdivo) and ipilimumab (Yervoy) previously demonstrated a significant benefit in OS compared with sunitinib and a manageable safety profile in the first-line setting for patients with advanced/ metastatic RCC in the phase 3 CheckMate 214 trial (NCT02231749).19 There is evidence suggesting that anti–PD-(L)1 monotherapy also results in clinical activity in patients with advanced RCC. This encouraged the development of the phase 3, double-blind CheckMate 914 trial (NCT03138512) to evaluate nivolumab plus ipilimumab in the early-stage adjuvant RCC setting in patients with a high risk of relapse after nephrectomy (FIGURE 1).20
The ongoing CheckMate 914 trial includes patients who have undergone partial or radical nephrectomy with negative surgical margins, predominantly clear cell histology, and no macroscopic residual disease or distant metastases post nephrectomy. Part A of the study will compare nivolumab plus ipilimumab vs placebo. Part B will compare 3 arms: nivolumab plus ipilimumab vs placebo vs nivolumab alone. The primary end point is DFS, and the total target enrollment is 1600 patients.21
Nivolumab in the Perioperative Setting: PROSPER Trial
Nivolumab is also being studied as monotherapy in PROSPER RCC (NCT03055013), an unblinded, phase 3 National Clinical Trials Network study. The concept behind this study is to prime the immune system with nivolumab prior to nephrectomy and then continue with adjuvant nivolumab therapy. Eligible patients have high-risk RCC of clinical stage T2 or greater or any T stage with regional lymph node involvement of any histology with plans to undergo radical or partial nephrectomy. Patients in the investigational arm will receive 1 dose of nivolumab prior to surgery, followed by 9 adjuvant doses spaced 4 weeks apart, whereas the control arm will undergo nephrectomy followed by surveillance.21
Durvalumab and Tremelimumab: RAMPART Trial
A UK-led multiarm, multistage platform trial, which may accommodate additional arms addressing new research questions over time, has just initiated. To be eligible for RAMPART (Renal Adjuvant Multiple Arm Randomized Trial; NCT03288532), patients must have histologically proven, resected, locally advanced RCC (both clear and non–clear cell types); and be at high or intermediate risk of relapse based on a Leibovich score between 3 and 11. Three arms are currently included.
Patients in arm A will be actively monitored for 1 year, patients in arm B will receive the durvalumab (Imfinzi) every 4 weeks for 1 year, and patients in arm C will receive the combination of durvalumab every 4 weeks for 1 year plus 2 doses of tremelimumab on day 1 of the first 2 cycles of durvalumab. DFS and OS are coprimary outcomes.22
Further Considerations for Adjuvant Therapy in RCC
McKay and Xu explained that patient selection will be an important consideration as more data are revealed concerning immuno-oncology in the adjuvant setting for patients with RCC. “The story with adjuvant therapy across cancer is overuse or overtreatment,” McKay pointed out. “By definition, you are going to treat some patients [whose cancer is] never going to recur and [who] are going to incur a toxicity which they would never have otherwise.”
“Some patients may have more benefit from adjuvant therapy than others. Perhaps patients with grade 4 high-grade disease or with rhabdoid or sarcomatoid features might have a greater benefit from adjuvant therapy,” Xu said. “Conversely, it may be that patients [with] oligometastatic [disease] at presentation [that is] completely resected may have a greater benefit.”
McKay also emphasized the importance of accurately and adequately determining the efficacy of these new adjuvant therapies. “Patients are living a lot longer with a lot more options for treatment in the metastatic setting. The overall survival data [do] need to be there, but it’s going to take almost 10 years to get.” Extended patient survival times present a challenge for rapidly obtaining OS data and can create difficulties in obtaining complete OS data through patient follow-up.
Additional questions remain regarding how to treat patients who relapse on or soon after adjuvant therapy. Xu emphasized that timing will likely play a large role in these cases. “A patient who relapses while on immunotherapy during that 1 year is theoretically unlikely to receive much benefit from second-line immunotherapy. On the other hand, there’s no reason to believe the VEGFR TKIs won’t be active in these patients. Then for someone who gets pembrolizumab and goes 2 to 5 years without recurrence, it is an open question as to if we can rechallenge with immunotherapy.”
Although the KEYNOTE-564 results and subsequent approval of pembrolizumab have been strongly encouraging for the field, they have also generated many more unanswered questions. Xu and McKay are encouraged and hope that trials will continue to show progress. “It’s positive for our patients [and] positive for [treatment of] the disease, and the hope is to cure more patients with RCC through adjuvant therapy in the future,” McKay said.
Rarer subtypes of RCC are also being evaluated (see “Recent Advances in Papillary Renal Cell Carcinoma,” page 30).
REFERENCES:
1. Swallow C. The management of residual disease after TKIs in GIST. Presented at: 2022 ESMO Sarcoma and GIST Symposium; January 31-February2, 2022; Virtual.
2. Mudan SS, Conlon KC, Woodruff JM, Lewis JJ, Brennan MF. Salvage surgery for patients with recurrent gastrointestinal sarcoma: prognostic factors to guide patient selection. Cancer. 2000;88(1):66-74. doi:10.1002/(sici)1097-0142(20000101)88:1<66::aid-cncr10>3.0.co;2-0
3. Rutkowski P, Nowecki Z, Nyckowski P, et al. Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. J Surg Oncol. 2006;93(4):304-311. doi:10.1002/jso.20466
4. An HJ, Ryu MH, Ryoo BY, et al. The effects of surgical cytoreduction prior to imatinib therapy on the prognosis of patients with advanced GIST. Ann Surg Oncol. 2013;20(13):4212-4218. doi:10.1245/s10434-013-3279-9
5. Du CY, Zhou Y, Song C, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer. 2014;50(10):1772-1778. doi:10.1016/j.ejca.2014.03.280
6. Xia L, Zhang MM, Ji L, Li X, Wu XT. Resection combined with imatinib therapy for liver metastases of gastrointestinal stromal tumors. Surg Today. 2010;40(10):936-942. doi:10.1007/s00595-009-4171-x
7. Guo Y, Liu J, Wang F, et al. The role of surgical resectionfollowing tyrosine kinase inhibitors treatment in patients with advanced gastrointestinal stromal tumors: a systematic review and meta-analysis. J Cancer. 2019;10(23):5785-5792. doi:10.7150/jca.30040
8. Rubió-Casadevall J, Martinez-Trufero J, Garcia-Albeniz X, et al; Spanish Group for Research on Sarcoma (GEIS). Role of surgery in patients with recurrent, metastatic, or unresectable locally advanced gastrointestinal stromal tumors sensitive to imatinib: a retrospective analysis of the Spanish Group for Research on Sarcoma (GEIS). Ann Surg Oncol. 2015;22(9):2948-2957. doi:10.1245/s10434-014-4360-8
9. Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive surgery for metastatic gastrointestinal stromal tumors treated with tyrosine kinase inhibitors: a 2-institutional analysis. Ann Surg. 2018;268(2):296-302. doi:10.1097/SLA.0000000000002281
10. Casali PG, Blay JY, Abecassis N, et al; ESMO Guidelines Committee, EURACAN, and GENTURIS. Gastrointestinal stromal tumours: ESMO–EURACAN–GENTURIS clinical practice guidelinesfor diagnosis, treatment and follow-up. Ann Oncol. 2022;33(1):20-33. doi:10.1016/j.annonc.2021.09.005
Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen