As the Oncology Care Model Expires, What’s to Become of the Biosimilar Market?
In surveys conducted between 2020 and 2021, Cardinal Health sought to determine oncologists’ perceptions about their use of biosimilars in their treatment regimens.

Although many oncologists are familiar with prescribing biosimilars, there awaits a changing landscape, as the Oncology Care Model (OCM) is set to expire in 2022. The OCM is a reimbursement model, where physicians assume greater financial risk for administered health care. No value-based care (VBC) substitution has yet been shared by the Center for Medicare and Medicaid Innovation, which may force oncologists to revert to prescribing familiar brands. It’s a significant development, as industry experts project biosimilars can reduce US drug expenditures by $133 billion by 2025.1
In an interview with Targeted Therapies in OncologyTM, Bruce Feinberg, DO, commented on the “2022 Biosimilars Report: The US Journey and Path Ahead,”2 giving insight on why biosimilars can still grow in clinical use among oncologists, what barriers there are to adoption and use of biosimilars, and what to expect come the expiration of the OCM.
In surveys conducted between 2020 and 2021, Cardinal Health sought to determine oncologists’ perceptions about their use of biosimilars in their treatment regimens. Of the 323 oncologists surveyed, 85% said they would prescribe biosimilars in indications that have been granted FDA approval based on extrapolation (FIGURE 12), 5% said they wouldn’t prescribe a biosimilar without clinical data to demonstrate its safety and efficacy, none of the doctors said they would not prescribe a biosimilar, and 10% were split between prescribing for only supportive care or only curative care.
Feinberg, the vice president of clinical affairs and chief medical officer at Cardinal Health in Atlanta, Georgia, explained how these numbers trend upward as physicians become more familiar with biosimilars over time. “The reason we see that 90% moving over is because [of] things that have happened [beyond just price differences] in the marketplace, in terms of payer policies, structural designs, [and] benefits, that are getting physicians to say, ‘Let me give this a try...in a patient population where I presume there is the least risk,’” Feinberg said.
A follow-up survey question asked oncologists how they felt about switching patients to biosimilars with the intent to cure. Most felt comfortable doing so if they were switching from a reference drug to a biologic (FIGURE 22). For curative intent, 25% said they would only prescribe to new patients.
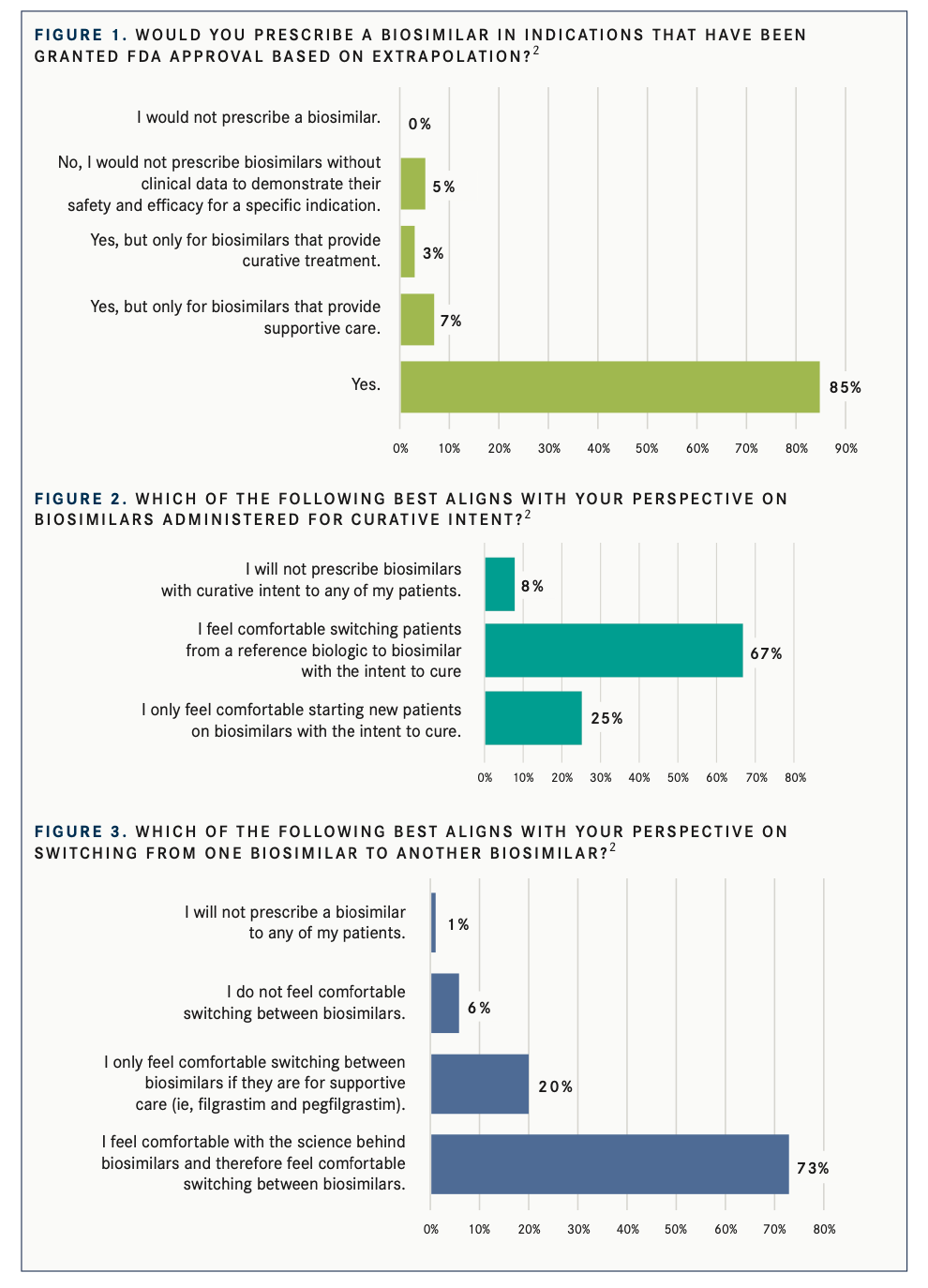
Oncologists were asked about switching from one biosimilar to another (FIGURE 32), and 73% felt comfortable and believed in the science behind biosimilars, 20% were only comfortable in supportive care circumstances, 6% did not feel comfortable switching biosimilars, and 1% said they would not prescribe any biosimilars.
“[With] more biosimilars coming to the market, it’s going to [potentially lead to] every year, your insurance company has contracted to use a different [drug] in the formulary,” Feinberg said, adding that it is important for physicians, government payers, and other stakeholders to understand that a branded system is flawed when biosimilars exist.
Feinberg explained that biosimilars have been trusted in the United States as a result of the OCM launched in 2016. Will doctors be more hesitant to prescribe biosimilars in the near future because insurance will not cover as much of the cost?
A comparative phenomenon occurred in Europe, where some countries have very high adoption rates, whereas others have rather low use of biosimilars in their practices. “If the policy embraced the biosimilar and rewarded the use of the biosimilar in some financial way, then it was used to a much greater extent than not,” Feinberg said.
A new VBC model is expected, but it may be several months after the expiration of the OCM. There is a level of uncertainty during this time, as physicians may have to adjust according to the costs of these biosimilars. Although this gap in time can seem volatile, a new care model is still expected. From what these surveys have shown, the use of these biosimilars will be trusted by physicians over time, as this will allow physicians to test them and become more comfortable with the science behind them.
REFERENCES:
1. Association for Accessible Medicines. The U.S. Generic & Biosimi- lar Medicines Savings Report: October 2021. Accessed February 15, 2022. https://bit.ly/3LzGKFE
2. Cardinal Health. 2022 Biosimilars Report: The U.S. Journey and Path Ahead. Accessed February 15, 2022. https://adobe.ly/3rQB- mWH
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More