Duvelisib Shows Encouraging Activity in Lymphoma
Duvelisib (IPI-145) is an orally administered, dual inhibitor of phosphoinositide 3-kinase (PI3K) delta (δ) and gamma (γ) isoforms, and the drug is selective for PI3K class I isoforms over other lipid and protein kinases.
1,2In preclinical studies, duvelisib inhibited survival of malignant B and T cells, with direct effects on tumor cells, and disrupted important tumor interactions with the microenvironment.3-5
In clinical studies, duvelisib effectively inhibited PI3K δ and γ when administered at doses of 25 mg twice daily or higher.6-8In addition to the activity shown in lymphoma, the drug has also shown promise in treating patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and other hematologic malignancies.8,9
At this year’s meeting of the American Society of Hematology (ASH) several presentations outlined novel preclinical and clinical findings with this agent.3,10-13
PI3K δ and γ: Critical Roles in Hematologic Malignancies
Duvelisib has inhibitory activity for both the PI3K δ and γ isoforms, although its inhibitory potency for the δ isoform is approximately 10-fold higher than that for the γ isoform.2The δ and γ PI3K isoforms show preferential expression in cells of the immune system, including T and B lymphocytes and myeloid cells. The isoforms have distinct and nonoverlapping roles in normal immune cell functions, including differentiation, activation, and migration.1,2
Given its inhibitory profile, duvelisib has been investigated in several hematologic cancers, including CLL, non- Hodgkin lymphoma (NHL), and a variety of myeloid malignancies.2,7-9,11,13The more limited expression of the δ and γ isoforms in lymphoid cells also suggests that duvelisib would be minimally toxic in other tissue types.1
Figure 1. Reduction in PD markers associated with the tumor microenvironment in B-cell malignancies3
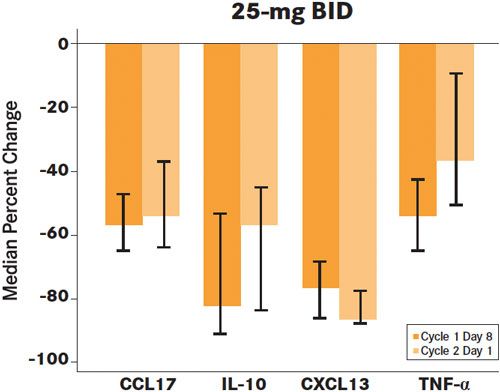
C1D8, n=13-16; n =12-13
Error bars: 25th and 75th percentiles
In a presentation at ASH 2014, Peluso and coinvestigators examined the effects of PI3K-δ,γ inhibition using duvelisib and 2 other inhibitors selective for the δ or γ isoforms individually.3In experimental models, these investigators demonstrated a number of activities attributable to PI3K δ or γ activity. For example, primary CLL cell proliferation induced by tumor microenvironment associated cytokines was found to be δ isoform dependent, whereas selective inhibition of the γ isoform could block macrophage polarization into the protumorigenic ‘M2’ phenotype, as opposed to the ‘M1’ phenotype, which inhibits cancer growth.3They further showed that M2-polarized macrophages enhance survival of CLL cells. In other experiments, they showed that T-cell migration and activation induced by CXCL12, an important tumor-associated chemokine, could also be blocked with PI3K γ inhibition. Collectively, the findings support the notion that dual PI3K-δ,γ inhibition with duvelisib can inhibit complementary pathways that are essential for the interaction of the tumor with its microenvironment.3
A Phase I Study in Patients With Indolent NHL
Also at ASH 2014, Flinn et al reported findings from a phase I study examining the use of duvelisib in patients with relapsed/refractory indolent NHL (R/R iNHL).10In this study, duvelisib was administered to 36 patients with iNHL at doses of 15 mg, 25 mg, 50 mg, and the maximum tolerated dose (MTD) of 75 mg twice a day. Based on the observed pharmacokinetic and pharmacodynamic (PK/PD) parameters and clinical activity, the 25-mg twice-a-day dose was selected for further phase II and III clinical studies.10The principal study endpoints included safety, adverse events (AEs), and clinical efficacy (patient response using revised International Working Group [IWG] criteria 2007), as well as exploratory endpoints such as PD parameters (changes in serum cytokines and chemokines). Subtypes of iNHL included follicular lymphoma (FL), small lymphocytic lymphoma (SLL), Waldenström’s macroglobulinemia (WM), marginal zone lymphoma (MZL), and not otherwise specified (NOS).
A total of 19 patients received the 25- mg twice-a-day dose (this included 1 patient who received 15 mg twice a day) with FL, n = 14; SLL, n = 4; and WM, n = 1. Most patients were Eastern Cooperative Oncology Group (ECOG) scale 0 or 1, and most had received 3 or more prior systemic therapies (58%).10The median time on treatment was 11.8 months, and 9 patients (47%) had received 12 or more months of duvelisib therapy. At the 25- mg twice-a-day dose, 14 patients (74%) discontinued treatment because of AEs (37%) and disease progression (21%).10
Several key PD markers associated with the tumor microenvironment in B-cell malignancies, including CCL17, interleukin (IL)-10, CXCL-13, and tumor necrosis factor (TNF)-alpha, showed modulation at the 25-mg twice-a-day dose; with the magnitude of reduction independent of dose and with reductions observed through cycle 2 day 1 (Figure 1).10At the 25-mg twice-a-day dose, the overall response rate (ORR) was 72% (n = 13 patients), with 33% of patients (n = 6) experiencing a complete response (CR); activity was observed across all of the iNHL subtypes, including the subgroup of patients with FL, which showed a 69% ORR and a 39% CR rate. The median time to response was 1.8 months (Table 1).10At the 25- mg dose, 13 of 17 (76%) had a nodal response, as dened by 50% or more reduction in adenopathy; progression- free survival (PFS) and overall survival (OS) in the 25-mg group was 69% and 89%, respectively, at 24 months, with a median PFS and OS not yet reached in the study.
Population
Pts
Best Response, n (%)
Median Time to Response, Months (Range)
n
CR
PR
M
SD
PD
ORR
25
18
6 (33)
6 (33)
1 (6)
4 (22)
1 (6)
13 (72)
1.8 (1.7, 5.5)
Table 1. Best Response in Patients with R/R iNHL Treated with Duvelisib 25-mg BID3
In the 25-mg twice-a-day group, the most commonly observed AEs, occurring in 20% or more of patients, included elevations in aspartate transaminase (AST) or alanine transaminase (ALT) (47%), cough (42%), nausea (37%), rash (37%), as well as diarrhea, pyrexia, and fatigue (each 32%). Grade 3 and 4 (Gr 3/4) AEs included AST or ALT elevations (37%), neutropenia (22%), pneumonia (16%), and diarrhea (16%). The most common serious AE occurring in >1 patient was pneumonia (21% at 25- mg dose). Overall, results of the study showed duvelisib to have encouraging activity with a rapidly observed response (median 1.8 months), and a tolerable safety prole, with most AEs being reversible, clinically manageable, and of grade 1 or 2.10
The clinical findings have prompted a phase II study (DYNAMO) in patients with R/R iNHL (NCT01882803), and a phase III study in combination with rituximab (DYNAMO+R) in patients with previously treated FL (NCT02204982), both of which are recruiting patients. In addition, a phase I/II trial in patients with previously untreated FL (CONTEMPO) is under way (NCT02391545).
In additional findings from ASH 2014, duvelisib has also shown promising activity in patients with R/R T-cell lymphoma,13in those with R/R CLL,11and in patients with CLL who have progressed on ibrutinib therapy.12
References
- Okkenhaug K. Two birds with one stone: dual p110δ and p110γ inhibition.Chem Biol. 2013;20(11):1309-1310.
- Winkler DG, Faia KL, DiNitto JP, et al. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models.Chem Biol. 2013;20(11):1364-1374.
- Peluso M, Faia K, Winkler D, et al. Duvelisib (IPI-145) inhibits malignant B-cell proliferation and disrupts signaling from the tumor microenvi- ronment through mechanisms that are depen- dent on PI3K-δ and PI3K-γ. 56th ASH Annual Meeting and Exposition. 2014. Abstract 328.
- Huang X, Proctor J, Yang Y, et al. The potent PI3K-δ,γ inhibitor, IPI-145, exhibits preclinical activity in murine and human T-cell acute lym- phoblastic leukemia. 55th ASH Annual Meeting and Exposition. 2013. Abstract 1438.
- Campbell V, Thompson R, Villegas V, et al. The potent PI3K-δ,γ inhibitor IPI-145 exhibits dif- ferential activity in diffuse large B-cell lymphoma (DLBCL) cell lines. 55th ASH Annual Meeting and Exposition. 2013. Abstract 1832.
- Kahl B, et al. Preliminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3- kinase-δ,-γ, in patients with relapsed/refractory B-cell lymphoma. 12th International Conference on Malignant Lymphoma. June 1922, 2013. Oral presentation #066.
- Horwitz SM, Flinn I, Patel MR, et al. Pre- liminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-δ,γ, in patients with relapsed/refractory lymphoma.J Clin Oncol. 2013;31(suppl): Abstract 8518.
- Patel MR, Kahl BS, Horwitz SM, et al. Pre- liminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-δ,γ, in patients with relapsed/refractory CLL.J Clin Oncol. 2013;31(suppl): Abstract 7070.
- IPI-145 shows promise in CLL patients. Cancer Discov. 2014;4(2):136. doi:10.1158/2159- 8290.CD-NB2013-177.
- Flinn I, Yasuhiro Oki Y, Manish Patel M. A phase 1 evaluation of duvelisib (IPI-145), a PI3K- δ,γ inhibitor, in patients with relapsed/refractory iNHL. 56th ASH Annual Meeting and Exposition. 2014. Abstract 802.
- O’Brien S, Patel M, Kahl BS, et al. Duvelisib (IPI-145), a PI3K-δ,γ inhibitor, is clinically active in patients with relapsed/refractory chronic lymphocytic leukemia. 56th ASH Annual Meeting and Exposition. 2014. Abstract 3334.
- Porcu P, Flinn I, Kahl BS, et al. Clinical activ- ity of duvelisib (IPI-145), a phosphoinositide-3- kinase-δ,γ inhibitor, in patients previously treated with ibrutinib. 56th ASH Annual Meeting and Exposition. 2014. Abstract 3335.
- Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinase-δ,γ inhibitor, shows activity in patients with relapsed/ refractory T-cell lymphoma. 56th ASH Annual Meeting and Exposition. 2014. Abstract 803.
Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen
