During ASCO, Studies Help to Refine CD19 CAR T-cell Use in Hematologic Malignancies
Several studies presented at the 2018 ASCO Annual Meeting helped further refine and inform treatment strategies for the budding class of CAR T-cell therapies, particularly those directed at CD19, in patients with hematologic malignancies, with a focus on predicting adverse events and optimizing efficacy.
Frederick L. Locke, MD
Treatment with chimeric antigen receptor (CAR) T-cell therapies continues to generate excitement as a promising immunotherapy approach, especially for patients with hematologic malignancies. Two CAR T-cell therapies now have FDA approval for use in blood cancers, and many clinical trials are continuing to improve upon successes seen with these agents.
Several studies presented at the 2018 ASCO Annual Meeting helped further refine and inform treatment strategies for the budding class of CAR T-cell therapies, particularly those directed at CD19, in patients with hematologic malignancies, with a focus on predicting adverse events (AEs) and optimizing efficacy.
In durability findings from the pivotal ZUMA-1 and TRANSCEND studies,1,2a 3-month cutoff was established for response, with more than 80% of responding patients continuing to stay in remission. Furthermore, findings from the phase I/II JCAR014 trial helped establish serum lactate dehydrogenase (LDH) levels pre-lymphodepletion as a signal of complete remission (CR), and a specific cytokine signature as a predictor of long-term outcomes.3,4
Other studies assessed patient populations for CAR T-cell trials to further establish treatment eligibility criteria. Although number of prior therapies did not seem to impact outcomes, prior treatment with an anti-CD19 therapy could alter the CAR T-cell manufacturing process, although the interpretation of data for this remains unclear.
In one scenario,5the anti-CD19 therapy blinatumomab (Blincyto) did not impact the manufacturing process for axicabtagene ciloleucel (axi-cel; Yescarta). However, in another dataset, more nonresponders and CD19-negative relapses were experienced by patients when blinatumomab was administered prior to CAR T-cell therapy.6
Jeremy S. Abramson, MD, MMSC
Adding to these efficacy-focused findings, data from the ROCKET study, which looked to further establish a patient profile to help predict toxicity with CAR T-cell therapies, were also presented at the ASCO meeting.7This analysis showed that patients with positive Philadelphia chromosome (Ph)-like genetic characteristics were highly unlikely to experience grade 4 or 5 cytokine release syndrome (CRS) or neurotoxicity.
DURABILITY OF RESPONSE
With the rapid develop of the CAR T-cell therapies, the durability of response and impact on long-term outcomes remains a question of interest, particularly as it factors into a value equation. As data from the clinical trials mature, it is becoming clear that patients experiencing a CR at month 3 to 6 are likely to experience a long-term remission.
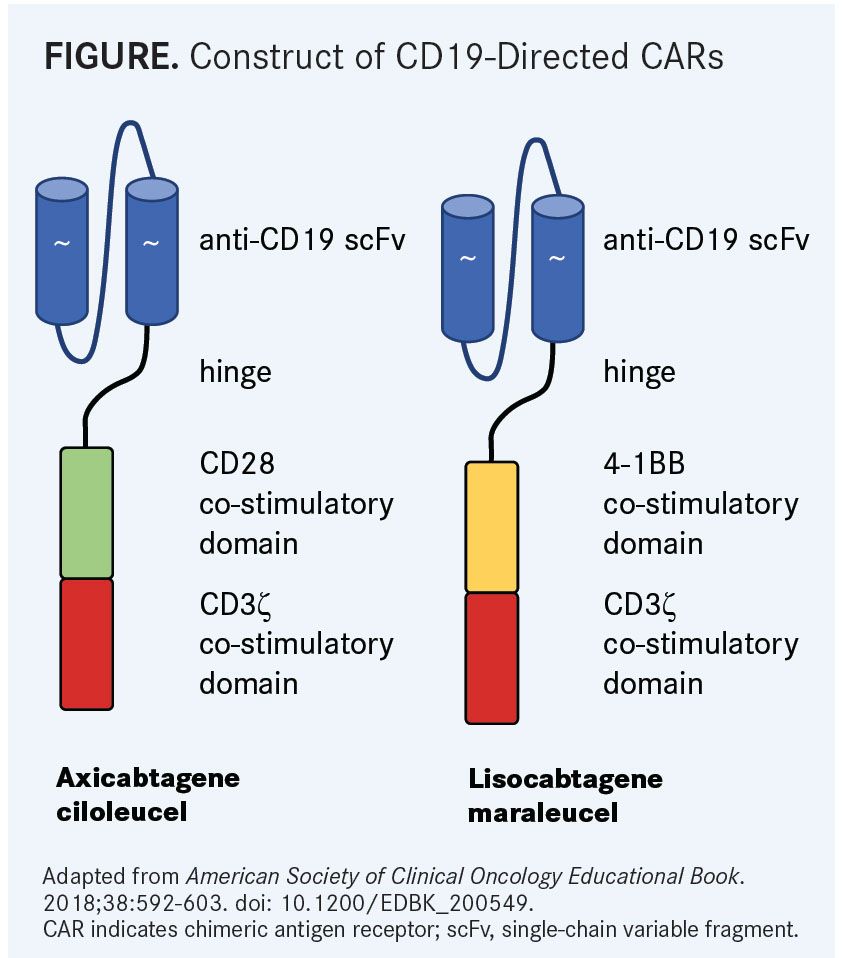
In the pivotal phase II ZUMA-1 study,1which is exploring the use of axi-cel (also known as KTE-C19), a CD19-directed CAR T-cell product with a CD28 costimulatory domain (FIGURE), in patients with refractory and aggressive non-Hodgkin lymphomas, 42% of responses to axi-cel remained ongoing after 15.4 months of follow-up. The median duration of response in the overall population (n = 108) was 11.1 months (95% CI, 3.9-not reached). In those with a CR, the median duration of response was not yet reached; however, patients achieving an initial partial remission (PR) had a median duration of response of just 1.9 months (95% CI, 1.4-2.1) (TABLE1,2). Additionally, the median overall survival (OS) had not yet been reached, but the 12-month OS rate was 60%.
The median time to response for axi-cel was 1 month (range, 0.8-14.8), and nearly half (41%) of the PRs transitioned to CRs as late as 1-year post infusion. At the 15.4-month follow-up, all but 1 response was a CR (40%).
The trial results showed that if any type of response was ongoing at month 3, there was an 80% likelihood of a continued response at 1 year. For those with a response at month 3 (9 with a PR and 42 with a CR), the progression-free survival (PFS) rate remained 78% at 6, 9, and 12 months for those with a PR. In the CR group, the rates were 88%, 83%, and 79% at months 6, 9, and 12, respectively. The median PFS had not yet been reached for both those who achieved a PR and for those who had a CR.
“Over half of progression events occurred by month 3, leading to the need to define treatment practice for patients at this time point. We believe that the month 3 time point is clinically relevant,” said lead investigator Frederick L. Locke, MD, program co-leader for immunology, and a translational researcher in the Department of Blood and Marrow Transplant and Cellular Immunotherapy, at the Moffitt Cancer Center. “Response to axi-cel, either a partial response or a complete response by 3 months, may be prognostic for long-term remission.”
These findings were echoed in the TRANSCEND trial, which is examining lisocabtagene maraleucel (JCAR017; liso-cel), using CD19-(4-1BB) CARs (FIGURE), in patients with high-risk diffuse large B-cell lymphoma (DLBCL).2Among the core group of patients (n = 73), consisting of patients with de novo DLBCL or DLBCL transformed from follicular lymphoma and patients with double- or triple-hit lymphoma, 88% who achieved a CR at 3 months continued to have a CR at month 6. Ninety-three percent of patients with a CR at 6 months continued to have an ongoing response.
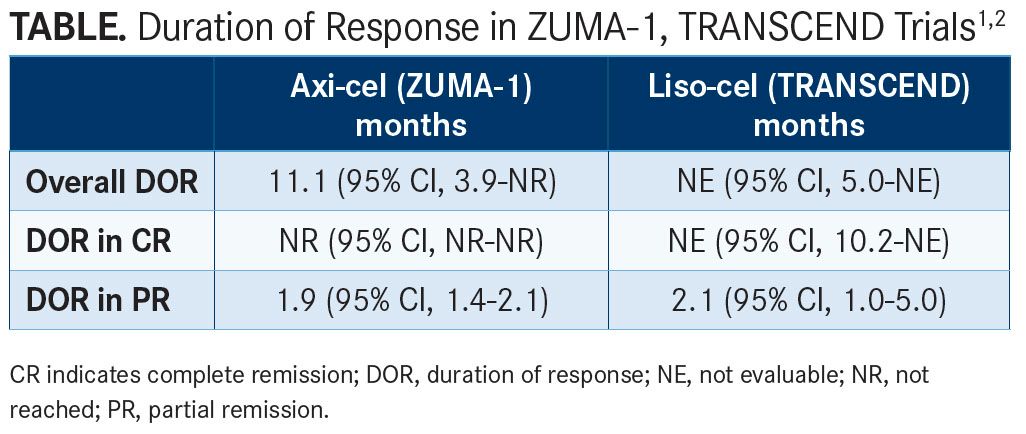
With a median of 8 months of follow-up, the median duration of response from the core population in those achieving a CR was not yet reached (95% CI, 10.2-not evaluable [NE]). Those with a PR had a median duration of response of 2.1 months (95% CI, 1.0-5.0) (TABLE1,2).
The overall 12-month OS rate was 63% (95% CI, 49%-74%) and in those achieving a CR, the 12-month OS rate was 89% (95% CI, 72%-96%). In those with a PR, the median OS was 10.3 months (95% CI, 6.8-NE) and the 1-year OS rate was 33% (95% CI, 9%-60%).
“The duration of response curves flatten out after the 3-month point, in both the full and the core datasets,” said lead investigator Jeremy S. Abramson, MD, MMSc, director of the Lymphoma Program at the Massachusetts General Hospital Cancer Center.
PREDICTING DEPTH OF RESPONSE
Focus has also shifted toward predicting which patients will reach a CR, as it implies a longer-term remission, with some patients alive several years following infusion. Several baseline levels have emerged as predictive.
In a study looking at JCAR014 in 57 patients with relapsed/refractory B-cell malignancies,3 lower pre-lymphodepletion serum LDH and higher peak CD8-positive CAR T cells were associated with better response and outcomes. In a multivariable logistic regression model, serum LDH levels prior to lymphodepletion correlated with CR (odds ratio [OR], 0.51; 95% CI, 0.26-0.90; P = .03) and higher peak CD8-positive CAR T cells at day 28 were associated with better PFS (HR, 0.81; 95% CI, 0.68-0.95; P = .01).
Moreover, a higher peak of cytokine interleukin (IL)-7 and a lower level of IL-18 at day 0 were associated with better PFS in patients who achieved a CR. In patients with a CR with high peak IL-7 and low level IL-18 at day 0, the 24-month PFS rate was 100% (P = .002).
In another study looking at patients with relapsed or refractory CD19-positive B-cell malignancies,4normal LDH levels and platelet counts ≥100 prior to cyclophosphamide/fludarabine (Cy/Flu) lymphodepletion were associated with better OS and disease-free survival (DFS). These patients, who were classified as good risk, had a 24-month DFS rate of 78% and 24-month OS rate of 86%. Regardless of risk, however, the analysis found that receiving a subsequent allogeneic hematopoietic stem cell transplant after CAR T-cell therapy also improved outcomes, with a 24-month DFS rate of 61% and a 24-month OS of 72%.
“In those who achieved minimal residual disease [MRD]-negative CR, multivariable modeling identified patients with lower LDH and higher platelet count prior to lymphodepletion who received Cy/Flu as having longer DFS,” said lead investigator Kevin Hay, MD, a postdoctoral research fellow at the Fred Hutchinson Cancer Research Center. “In patients with MRD-negative CR after CD19 CAR-T cells, allogeneic hematopoietic stem cell transplantation may improve DFS/OS. This potential benefit appears to exist in both good-risk and bad-risk patients identified from the multivariable modeling.”
IMPACT OF PRIOR BLINATUMOMAB
Results were mixed on the impact of prior treatment with blinatumomab on response to treatment with CAR T-cell products.5,6An analysis from the ZUMA-3 trial of axi-cel in adult patients with relapsed/refractory acute lymphoblastic leukemia (ALL),5suggested that prior treatment with blinatumomab did not significantly alter results, whereas findings from another study from Children’s Hospital of Philadelphia (CHOP)6 showed that prior blinatumomab resulted in more nonresponses and higher CD19-negative relapses.
In the CHOP analysis,6cases were reviewed for 150 patients aged 1 to 30 with B-cell ALL (B-ALL) who were treated with CAR T-cell therapy. Overall, 8% of patients were nonresponders and 21.33% had CD19-negative relapses. Across the full population, 10.67% had received prior blinatumomab. Thirteen percent of patients had diminished CD19 expression at baseline, which was more commonly seen following treatment with blinatumomab.
A higher proportion of responders (~60%) had not received a prior CD19-targeted therapy compared with blinatumomab-treated patients (~32%). Additionally, more patients pretreated with a CD19-targeted agent had no response or a CD19-negative relapse (~60%) compared with non-pretreated patients (~28%).
The study authors, led by Vinodh Pillai, MD, PhD, suggested in their poster that the “benefits of non-curative CD19-targeted therapy must be carefully weighed against the possibility of nonresponse to curative CAR T-cell therapy.”
In the ZUMA-3 trial,5approximately half of the 23 treated patients had received prior blinatumomab before treatment with axi-cel. The median age was 34 years in the prior blinatumomab group and 49 years in the untreated arm. The median blast level was higher in the blinatumomab-pretreated patients versus untreated (85 vs 66). Additionally, a smaller overall CAR T-cell dose was manufactured for the pretreated group.
Similar rates of CR and MRD negativity were achieved in those treated and untreated with prior blinatumomab, according to investigator Bijal D. Shah, MD. The CR rate in the prior-blinatumomab group was 63% versus 80% in the untreated arm. MRD-negativity rates were 88% and 100%, favoring the naive group. There was no significant increase in grade ≥3 neurologic events between the groups.
“Prior blinatumomab did not impact the successful manufacture of KTE-C19. Clinical benefit was observed for patients with CD19-positive relapsed/refractory ALL, regardless of prior blinatumomab,” said Shah, a medical oncologist from Moffitt Cancer Center. “Results support continued study of KTE-C19 as an effective treatment option for adult relapsed/refractory ALL regardless of prior exposure to CD19-directed therapy.”
MOLECULAR SUBTYPE PREDICTS AES
Research efforts have focused extensively on reducing the frequency and severity of AEs associated with CAR T-cell therapies, with a distinct focus on CRS and neurotoxicity. Although treatment algorithms that include the use of the IL-6 inhibitor tocilizumab (Actemra) for CRS and corticosteroids for neurotoxicity have helped ameliorate some of these events, concerns are still present.
The ROCKET study of JCAR015 in patients with relapsed/refractory B-ALL was discontinued in March 2017 due to 5 patient deaths from cerebral edema. However, the trial continues to be analyzed to inform future studies using CAR T-cell therapy and to prevent such toxicity rates and severity.
In a gene expression analysis of the ROCKET study, 119 genes were differently expressed between those with grade 0/1 neurotoxicity and patients with grade 4/5 events. The grade 0/1 genes were more commonly found in Ph-positive or Ph-like samples, whereas grade 4/5 events occurred in nonPh-like samples.
When looking to patients across 4 clinical trials (ROCKET, MSK09-114 [NCT01044069], FHCRC2639 [NCT01865617], and PLAT-02 [NCT02028455]), 107 of 124 patients (86.3%) had grade 0 to 3 neurotoxicity in the nonPh-positive/Ph-like group. Additionally, 17 of 124 patients (13.7%) had a grade 4 to 5 event. In the Ph-like or Ph-positive group, all patients had neurotoxicity of grade 0 to 3 but none had a grade 4 to 5 event.
As a potential mechanism for these differences, chemokine CCL17, which is involved in trafficking and activation of regulatory and Th2 T cells, was more commonly expressed in tumors from patients with grade 0 or 1 neurotoxicity and has also been found to be highly expressed in Ph-like B-ALL.
“This identified a neurotoxicity-associated gene set that separates B-ALL based on molecular subtype,” said presenter Jae H. Park, MD, a hematologic oncologist in the Leukemia Service at Memorial Sloan Kettering Cancer Center. “These findings suggest that patient risk stratification by molecular subtype of disease and/or gene expression signature could play an important role in identifying patients at elevated risk for neurotoxicity.”
References:
- Locke FL, Ghobadi A, Jacobson CA, et al. Durability of response in ZUMA-1, the pivotal phase 2 study of axicabtagene ciloleucel (Axi-Cel) in patients (Pts) with refractory large B-cell lymphoma. J Clin Oncol. 2018;36(suppl; abstr 3003). meetinglibrary.asco.org/record/159145/abstract.
- Abramson JS, Gordon LI, Palomba ML, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol. 2018;36(suppl; abstr 7505). meetinglibrary.asco.org/record/159487/abstract.
- Gauthier J, Hirayama AV, Hay K, et al. Factors associated with duration of response after CD19-specific CAR-T cell therapy for refractory/relapsed B-cell non-Hodgkin lymphoma. J Clin Oncol. 2018;36(suppl; abstr 7567). meetinglibrary.asco.org/ record/162353/abstract.
- Hay K, Gauthier J, Hirayama AV, et al. Factors impacting disease-free survival in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR-T cells. J Clin Oncol. 2018;36(suppl; abstr 7005). meetinglibrary.asco.org/record/161626/abstract.
- Shah BD, Oluwole OO, Baer MR, et al. Outcomes of patients (pts) treated with prior blinatumomab (Blin) in ZUMA-3: A study of KTE-C19, an anti-CD19 chimeric antigen receptor (CAR) t cell therapy, in adult pts with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2018;36(suppl; abstr 7006). meetinglibrary.asco.org/record/163937/abstract.
- Pillai V, Rosenthal J, Muralidharan K, et al. Correlation of pre-CAR CD19 expression with responses and relapses after CAR T cell therapy. J Clin Oncol. 2018;36(suppl; abstr 3051). meetinglibrary.asco.org/record/161830/abstract.
- Olson NE, Ragan SP, Ponko S, et al. Tumor gene signature associated with neuro-toxicity in R/R B-ALL patients treated with JCAR015, a CD19-directed CAR T cell product. J Clin Oncol. 2018;36(suppl; abstr 7007). meetinglibrary.asco.org/ record/162487/abstract.
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
