Comparing Targeted Therapies in Infiltrative Basal Cell Carcinoma
At a live virtual event, Deborah J. Wong, MD, PhD, gave her thoughts on the use of hedgehog inhibitors compared with immunotherapy in patients with infiltrative basal cell carcinoma.
Deborah J. Wong, MD, PhD
Associate Clinical Professor of Medicine
Hematology Oncology
University of California, Los Angeles
Los Angeles, CA

At a live virtual event, Deborah J. Wong, MD, PhD, gave her thoughts on the use of hedgehog inhibitors compared with immunotherapy in patients with infiltrative basal cell carcinoma. Both drug classes come with their own advantages and disadvantages, but the increasing use of anti–PD-1 therapy in this patient population, along with potentially shrinking the tumor prior to surgery, looks to create a paradigm shift. Wong discussed these points and the data behind them, and highlighted the continuing need for physicians to consider the patient holistically by working with a multidisciplinary care team.
INHIBITORS IN COMPARISON WITH ANTI–PD-1 THERAPY
WONG: The hedgehog pathway inhibits the Smoothened protein, which then affects downstream targets.1 There are 2 hedgehog inhibitors that are FDA approved, which are vismodegib [Erivedge] and sonidegib [Odomzo].2,3 Vismodegib is approved [to treat] patients with locally advanced and metastatic basal cell carcinoma.
The label for sonidegib [is indicated for patients with] locally advanced basal cell carcinoma. These are daily drugs at the starting dose and [have several adverse events (AEs)] that many physicians will be familiar with. Technically, cemiplimab-rwlc [Libtayo] was studied [as] opposed to hedgehog inhibitors in patients who, for some reason, were not eligible for hedgehog inhibitors,4 but we don’t know what the ideal sequence of [these drugs] is.
The benefit of a hedgehog inhibitor is that we can have quick responses as with many targeted therapies, but of course the downside is the development of resistance and their level of tolerability, which we also see with targeted therapies. The PD-1 [inhibitors] also have unique [toxicity] profile, but patients may have a prolonged response to them. We formally don’t know what the optimal sequence is, and [for now], we just have the approval guidelines.
In my practice, in general, if a patient is open to the idea of systemic therapy, I [find] that it’s an easier sell with immune therapies. I think [this is] because there’s so much in the [nonmedical] press about immunotherapies. The cases in which patients are more open to a hedgehog inhibitor would be if they have limited resources to come into the office and/or limited support [at home]. Then they might prefer an oral medication.
EFFICACY AND SAFETY WITH HEDGEHOG INHIBITORS
With vismodegib, in the locally advanced setting, we have an overall response rate [ORR] of 43% [compared with] 30% in the metastatic space.5 For sonidegib, in the locally advanced setting, the ORR is 44% and was lower in the metastatic space, so it’s only approved for [patients with] locally advanced disease.6 The median progression-free survival [PFS] for vismodegib is 23 months,5 so it can be prolonged, but the issue here is whether patients can tolerate it and will resistance develop, [because] there are several different resistance mechanisms that have been identified to date. So can we see prolonged responses to hedgehog inhibitors?
There was a small study that followed patients who had been on vismodegib for 8 months and then discontinued vismodegib.7 [Findings from] that small study demonstrated...a 3-year relapse-free survival in about one-third of patients, so 36%, with an 85% ORR if they were subsequently rechallenged.7 So there are cases in which patients who continue hedgehog pathway inhibitors may experience a prolonged response, but development of acquired resistance is a big issue. Again, one of the main issues with hedgehog inhibitors is the toxicities.... About one-third of patients will have gastrointestinal toxicities, including nausea, diarrhea, constipation, and vomiting.8 Fatigue is also a big issue, with 40% [of patients on vismodegib having] some degree of fatigue, with 5% [of cases] being grade 3. Weight loss and decreased appetite [are notable issues] as well, leading to severe grade 3 or worse toxicity in 7% of patients. Muscle spasms are a unique adverse event [because] we don’t commonly see it, [but here], almost 72% [of patients] had muscle spasms, with 3.6% [having] grade 3 arthralgias. Another unique AE we don’t commonly [see but do see with hedgehog pathway inhibitors is] dysgeusia and arthralgias, so a loss of taste or altered taste, which in the setting of decreased appetite and weight loss presents additional challenges for patients to maintain weight, and it will certainly affect their quality of life.8
EFFICACY AND SAFETY WITH CEMIPLIMAB
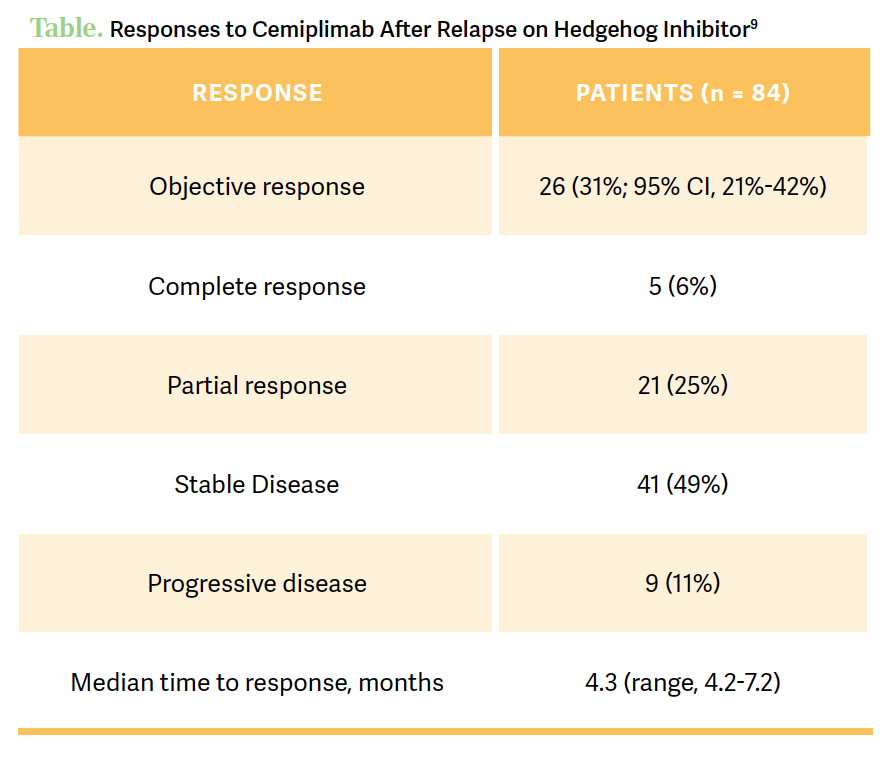
The 6-month PFS was 76%, and the 12-month [PFS] was 77%, but a median overall survival [OS] and duration of response had not been reached [Table9] However, the 2-year OS rate was 80%, which was quite high. In terms of treatment-related AEs [TRAEs], most patients had at least 1 TRAE, but about half of these [51%] were deemed grade 3 or worse. In terms of toxicities leading to discontinuation, [they] were seen in 17% [of patients in the trial given cemiplimab], but serious toxicities greater than or equal to grade 3 [were seen in] 8%, and 3 deaths were associated with treatment. The most common AEs associated with cemiplimab were consistent with what we see for AEs with anti–PD-1 agents. Again, [it was] mainly mild fatigue [26%] and about a quarter of patients had diarrhea [24%], but pruritus [21%] is a big one [whereas] asthenia [19%], decreased appetite [14%], and hypothyroidism [10%] are to be expected.9
APPROACHING CARE BASED ON THE PATIENT’S DESIRES
Using these systemic therapies as a neoadjuvant-type treatment approach is where it makes a lot of sense. That’s where the strength of the multidisciplinary team and consultative services input is very important.
One question I always ask the surgeons, or they’ll even bring it up, is whether the local therapy happening at surgery would change [the surgery] based on tumor shrinkage with a systemic therapy. And depending on the surgeon, some of them will still worry about the original margins and defects, but it’s a reasonable thing to consider in someone who otherwise would not have a local therapy option. However, there aren’t any data to date for these therapies in the adjuvant setting, but it is being evaluated. That is a very important question, because patients who have high-risk basal cell carcinoma tend to develop recurrent disease more.
Thinking about palliative surgery or palliative local therapy to preserve patients from the toxicities of long-term systemic therapy can work both ways. Sometimes we offer systemic therapies to limit the morbidity of the local therapies, but more and more, a lot of our multidisciplinary discussions in the setting of toxicity [are about] systemic therapies.... At some point, being able to offer a local therapy [such as] radiation or surgery, even if it’s not going to completely clear the disease, can buy time to give patients a break [from] systemic treatments at the very least.
REFERENCES
1. Harris L. Basal cell carcinoma: a pharmacist’s guide. US Pharm. 2019;44(8):29-35.
2. Axelson M, Liu K, Jiang X, et al. U.S. Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19(9):2289-2293. doi:10.1158/1078-0432.CCR-12-1956
3. Kish T, Corry L. Sonidegib (Odomzo) for the systemic treatment of adults with recurrent, locally advanced basal cell skin cancer. P T. 2016;41(5):322-325.
4. Stratigos AJ, Sekulic A, Peris A, et al. LBA47 primary analysis of phase II results for cemiplimab in patients (pts) with locally advanced basal cell carcinoma (laBCC) who progress on or are intolerant to hedgehog inhibitors (HHIs). Ann Oncol. 2020;31(suppl 4):S1175-S1176. doi:10.1016/j.annonc.2020.08.2277
5. Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17(1):332. doi:10.1186/s12885-017-3286-5
6. Villani A, Fabbrocini G, Costa C, Scalvenzi M. Sonidegib: safety and efficacy in treatment of advanced basal cell carcinoma. Dermatol Ther (Heidelb). 2020;10(3):401-412. doi:10.1007/s13555-020-00378-8
7. Słowińska M, Dudzisz-Śledź M, Sobczuk P, et al. Analysis of efficacy and safety of vismodegib therapy in patients with advanced basal cell carcinoma - real world multicenter cohort study. J Eur Acad Dermatol Venereol. 2022;36(8):1219-1228. doi:10.1111/jdv.18070
8. Erivedge. Prescribing information. Genentech; 2023. https://tinyurl.com/68vhzbc3
9. Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):848-857. doi:10.1016/S1470-2045(21)00126-1

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More