Anticipating Onset of Adverse Events Related to Lenvatinib/Pembrolizumab in Endometrial Cancer
During a Targeted Oncology™ Case-Based Roundtable™ event, Matthew A. Powell, MD, discussed management of adverse events with the combination of lenvatinib plus pembrolizumab in patients with endometrial cancer.
Matthew A. Powell, MD
Professor of Obstetrics and Gynecology
Chief, Division of Gynecologic Oncology,
Washington University School of Medicine Siteman Cancer Center
St Louis, MO

CASE SUMMARY
- A 71-year-old postmenopausal woman presented with abnormal uterine bleeding, increasing urinary frequency, and nausea/cramping for approximately 6 months.
- She has 2 grown children and no known family history of cancer.
- Her body mass index is 32; she has had type 1 diabetes since childhood, well controlled with medication.
- Physical examination was notable for enlarged uterus and right lower quadrant abdominal tenderness on palpation.
- ECOG performance status: 1
- CT of chest, abdomen, and pelvis showed uterine and bladder masses .
- Cancer antigen 125 (CA-125) level: 38.6 U/mL
- Endometrial biopsy: endometrial adenocarcinoma, International Federation of Gynecology and Obstetrics stage IVA, grade 3 (poorly differentiated)
- Notable molecular features: mismatch repair proficient (pMMR), microsatellite stable, estrogen receptor negative, HER2 negative, NTRK negative
- Germline testing showed no pathogenic variants.
- Carboplatin/paclitaxel chemotherapy was initiated; treatment was well tolerated other than grade 1 peripheral neuropathy and vomiting.
- Four months after initiation, she had no evidence of disease on PET scan, with complete resolution of symptoms.
- Twelve months post chemotherapy, rising CA-125 level was documented.
- CT scan of the abdomen and pelvis showed growth of bladder mass and new suspicious peritoneal lymph nodes .
- PET scan: intense fluorodeoxyglucose F 18–avid lesions in lungs, peritoneum, as well as para-aortic lymph nodes
- Bronchoscopy with biopsy revealed endometrial carcinoma cells.
- The patient received lenvatinib (Lenvima) plus pembrolizumab (Keytruda) as second-line therapy.
What are the most significant adverse events (AEs) to watch for in patients receiving lenvatinib and pembrolizumab for pMMR advanced endometrial cancer?
POWELL: A lot of us…want to learn more about how to manage the AEs. Both agents cause hypothyroidism. [It is associated with] pembrolizumab and multitargeted tyrosine kinase inhibitors [TKIs], so it happens a lot, and we monitor for it.1,2 We are quick to treat hypothyroidism and manage it.
I think [we are] quite comfortable with managing hypothyroidism if you use any of these agents. Hypertension is probably the bigger [concern], and it is key to get that patient’s [hypertension] under control before you even start the drug. Make sure their blood pressure is controlled, [and] make sure they know they need frequent monitoring because hypertension can happen early and fast. That is definitely something that we all should consider with [this regimen].
Nausea is something I think most [oncologists] are used to managing. Diarrhea is the one that makes us nervous. [Does the patient have] an immune-related colitis, or do they have just TKI-induced diarrhea?
The key there is to hold the TKI when they get diarrhea—and start even earlier than that. Tell them, “You need to notify us when you get diarrhea. You can take some initial antidiarrheals, but we need to know when this gets to be a problem because we need to have you hold your TKI initially.” If that doesn’t resolve, we need to think [it could be] immune-related colitis because it usually resolves quite quickly [after] stopping lenvatinib if it’s related to lenvatinib.
How long after initiation of treatment is the onset of treatment-related AEs in these patients?
[There are data on the time to first onset of] adverse reactions to lenvatinib/pembrolizumab [Table 13]. Hypertension happens early, [at a median of] 2.1 weeks.3 Fatigue tends to happen very early [median, 2.3 weeks], but once they get through that initial period, that fatigue is much [better] managed. Getting them on the proper dose is also important. They will have some musculoskeletal pains and issues there.
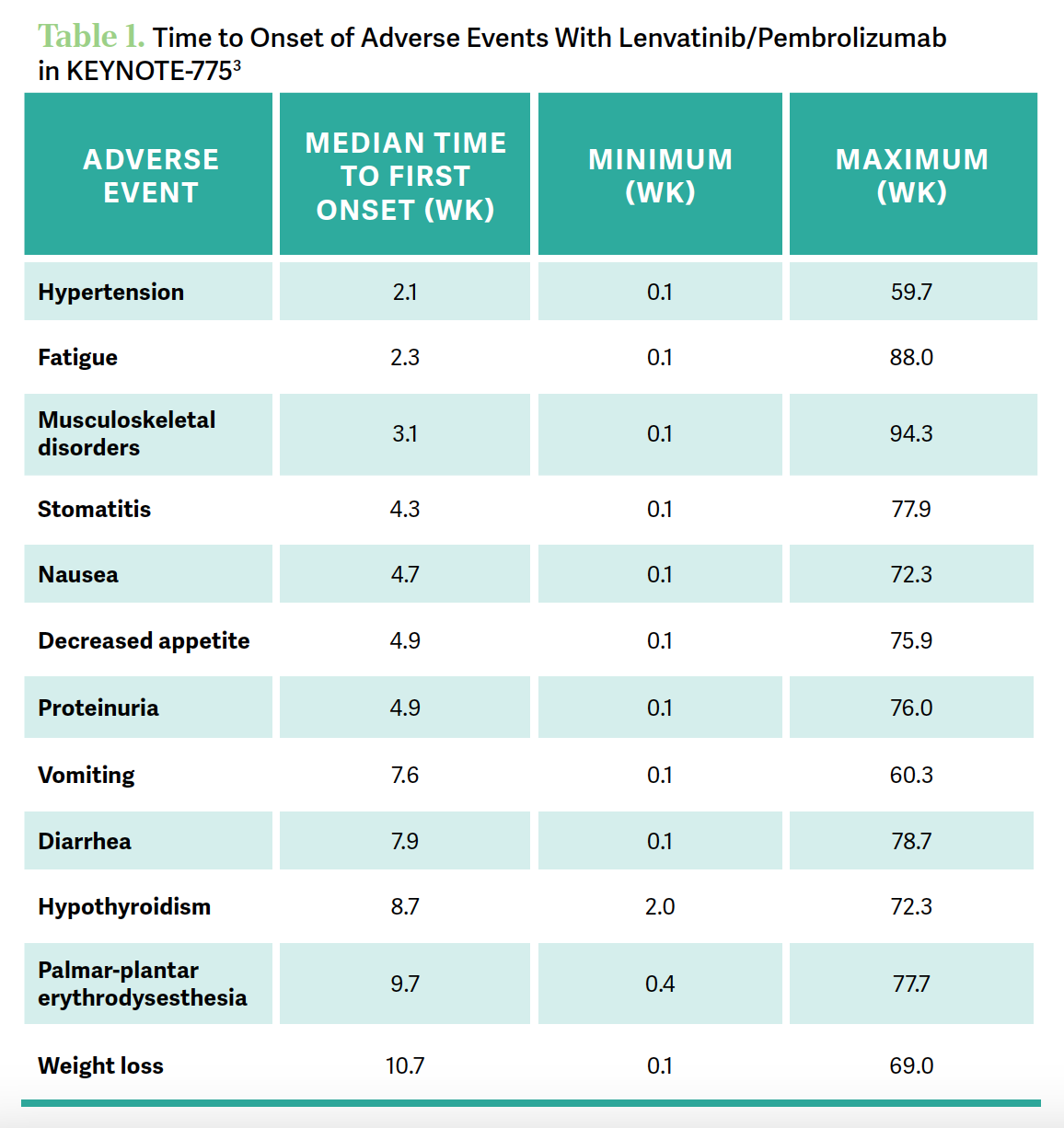
When you look at diarrhea, onset at about 8 weeks is the median—it can happen fairly early, but that’s the median onset—and again…usually if it’s an earlier onset, it’s probably the TKI, [but] if it’s later onset, it’s more likely immune related. That’s not a perfect [guideline], but keep that in mind. For hypothyroidism, the onset is often [at approximately] 9 weeks.
Palmar-plantar erythrodysesthesia can certainly happen as well. Although it’s typically not as bad as it is with liposomal doxorubicin, it is something to take note of. Proteinuria can be a problem with all the VEGF drugs, and it’s certainly something that could lead to discontinuation if that gets to be a problem.
[These data were discussed] by Nicoletta Colombo, MD, PhD, [et al] in The Oncologist in 2023.3 It has a very nice article looking at all the factors that I just mentioned and how to treat and manage [them]. What to do is very well spelled out in this manuscript, looking at managing the big areas there.
What are the practical considerations for dosing of lenvatinib in patients with endometrial cancer?
The starting dose for lenvatinib is 20 mg [daily].1 Most patients probably end up on 14 mg. Dose holds, dose delays, and dose reductions are very common, and I wouldn’t be discouraged if you have to do those things. That’s part of the management of TKIs, and these patients typically have more diabetes, or are obese, and perhaps had radiation therapy.
All of those are factors that go into dosing strategies. Be quick to do dose holds, dose delays, and dose reductions to get to the appropriate dose. The starting dose of 20-mg [lenvatinib daily] and 200-mg [pembrolizumab every 3 weeks] is what the [KEYNOTE-775 study (NCT03517449)] did.4
There are patients who, if their hypertension isn’t quite as controlled as you’d like, [you would] start at a lower dose to ease the patient into it a little bit. That hasn’t been studied well.
There are some retrospective multi-institutional series that support that we maintain the efficacy [with a 14-mg dose].5 I typically try to start at the higher doses and quickly de-escalate if need be. There wasn’t [dose] escalation in the study, so we don’t know what those differences are, but I think everybody needs slightly different doses. Some patients do well with 20 mg, [and] we’ve always wished we could figure out why. But [because of] the fatigue, often patients may need a 5-days-on, 2-days-off strategy that’s employed a lot now with TKIs, and it does make it tolerable for these patients. Especially as they get further out and you have disease control, you can feel a little more comfortable about decreasing doses and allowing improvement in fatigue.
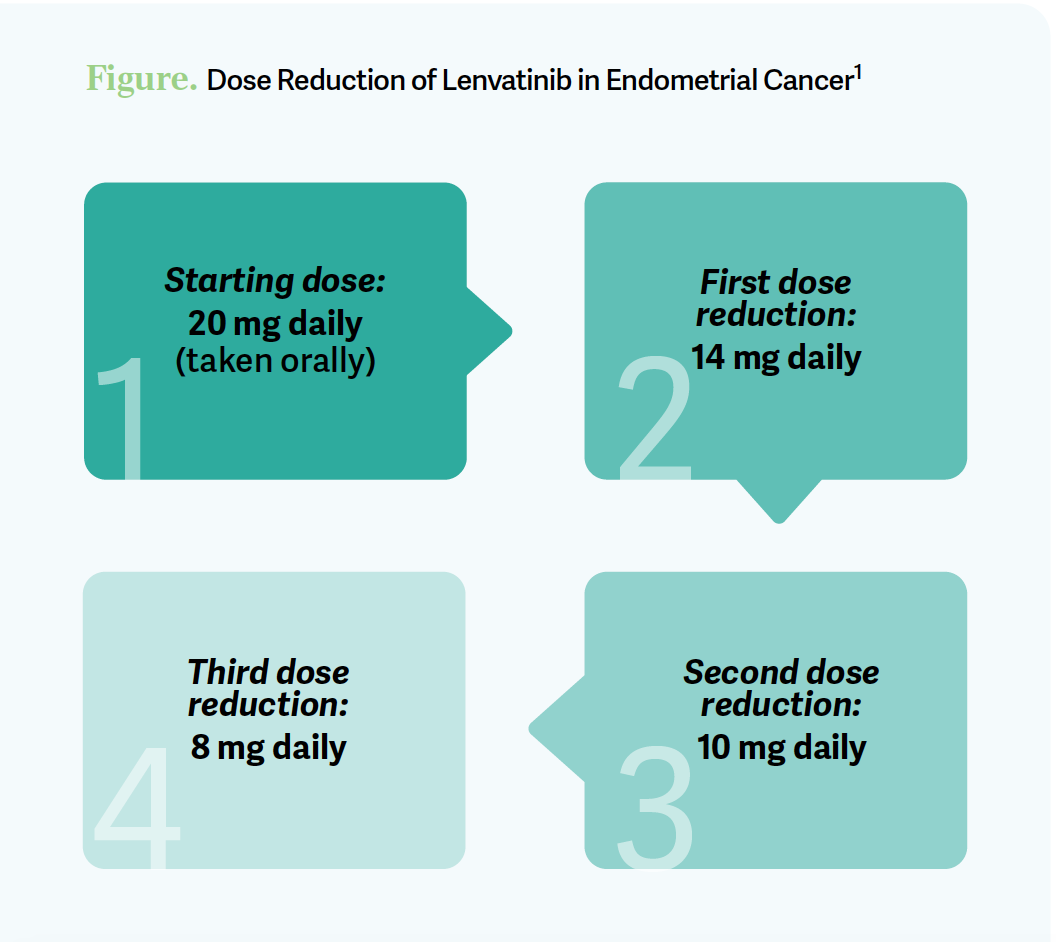
The dose strategy [is to decrease from] 20 mg to 14 mg, then 10 mg, then 8 mg [Figure1]. You can get to 18 mg, but this was what was on the study. [Doses of] 10 mg and 4 mg come in the capsules. I tell the patient when I’m starting, “If you have a lot of trouble right away, [we’ll] hold your lenvatinib; we’ll work on getting you on the right dose.” Oncologists don’t always like phone calls, but choose your patient, get them [ready], have your allied [health] professionals involved with the education and [communicate about] when they need to call [the oncologist]. Daily blood pressure monitoring is something we do; we get their blood pressure under good control to begin with and [do] the appropriate things when we’re using TKIs.
How does the dosing of lenvatinib in KEYNOTE-775 compare with what is used in other tumor types?
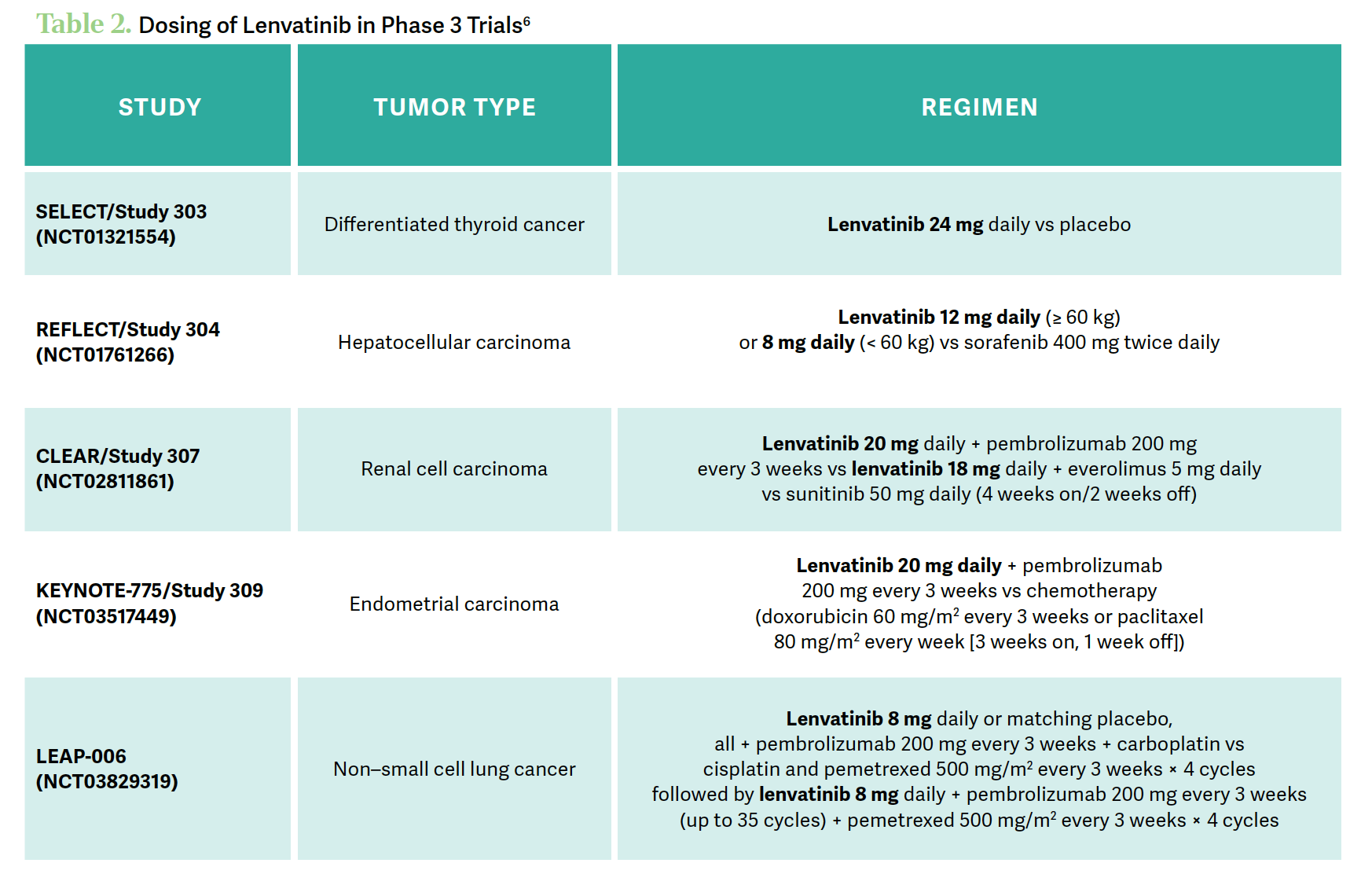
The CLEAR study [NCT02811861] of [patients with] renal cell carcinoma was one that was similar in dosing strategy [with] 200-mg [pembrolizumab] and 20-mg lenvatinib. Across the different studies, [they used] different doses of lenvatinib, but [we should be] trying to maintain that higher dose at least initially. There have been some weight-based dosing strategies involved in some of the other tumors [Table 26].
REFERENCES
1. Lenvima. Prescribing information. Eisai; 2022. Accessed November 7, 2023. https://tinyurl.com/bdz5z5fs
2. Keytruda. Prescribing information. Merck; 2023. Accessed November 7, 2023. https://tinyurl.com/rvy57ehu
3. Colombo N, Lorusso D, Monk BJ, et al. Characterization and management of adverse reactions in patients with advanced endometrial cancer receiving lenvatinib plus pembrolizumab. Oncologist. Published online July 31, 2023. doi:10.1093/ oncolo/oyad201
4. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437-448. doi:10.1056/ NEJMoa2108330
5. How JA, Patel S, Fellman B, et al. Toxicity and efficacy of the combination of pembrolizumab with recommended or reduced starting doses of lenvatinib for treatment of recurrent endometrial cancer. Gynecol Oncol. 2021;162(1):24-31. doi:10.1016/j.ygyno.2021.04.034
6. Motzer RJ, Taylor MH, Evans TRJ, et al. Lenvatinib dose, efficacy, and safety in the treatment of multiple malignancies. Expert Rev Anticancer Ther. 2022;22(4):383- 400. doi:10.1080/14737140.2022.2039123

2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.