Roundtable Roundup: Choosing Third-Line ccRCC Therapy
In separate live events, Yasser Mohamed Ali Ged, MBBS, and Deepak Kilari, MD, discussed the best treatment options for patients with advanced renal cell carcinoma and what role tyrosine kinase inhibitors play for these patients.
CASE SUMMARY
A 71-year-old man with a history of metastatic renal cell carcinoma (RCC) was post left nephrectomy and adrenalectomy with clear cell RCC (ccRCC) and metastases in the adrenal gland. Four years later he experienced disease recurrence, and lung nodules with biopsy were consistent with ccRCC. It was later recognized that the nodules had been present on scans over 2 prior years. The patient was observed based on low-volume and indolent behavior of disease and patient preference. However, 18 months later there was continued indolent growth observed on scans, increased total tumor burden, new paratracheal lymph nodes (2.0 × 1.5 cm), and multiple growing pulmonary nodules.
A decision was made to initiate systemic therapy with pembrolizumab (Keytruda) 200 mg intravenously every 3 weeks plus axitinib (Inlyta) 5 mg orally twice a day. Stable disease was seen on follow-up with moderate diarrhea (well controlled with antidiarrheal medication) and mild fatigue reported. He had no fever or recent sick contact, and his flu vaccine was up to date.
After treatment cycle 6, he developed fatigue, mild shortness of breath, and mild cough without chest pain. Pulse oxygen was 93% at rest. Infectious work-up was negative for bacterial, fungal, and viral organisms. A CT scan of the chest at week 18 confirmed grade 3 pneumonitis. Prednisone was administered at 50 mg orally once a day for 4 weeks followed by a taper of 10 mg every week for 4 weeks. Pembrolizumab was discontinued, and the patient continued axitinib.
Fourteen months after initiation of systemic therapy, the patient reported increasing back pain, mild nausea, weight loss, and new onset of persistent rib pain. Imaging confirmed disease progression with growth of the paratracheal lymph node (from 20 × 15 mm to 25 × 28 mm), new mediastinal and hilar nodal involvement, new retroperitoneal nodes, and new lytic osseous lesions. His ECOG performance status was 1. Cabozantinib (Cabometyx) was given at 60 mg once a day, but 9 months later, disease progression was documented. The patient would like to continue active therapy.
Yasser Mohamed Ali Ged, MBBS
Codirector, Kidney Cancer Research Program
Assistant Professor of Oncology
Johns Hopkins Medicine
Baltimore, MD

GED: The most recent National Comprehensive Cancer Network [NCCN] Kidney Cancer Panel changed the classification of second-line therapy by stratifying the subsequent therapies or for ccRCC based on whether the patient had previously received any immunotherapy [IO].1 Right now, patients are categorized into whether they are IO therapy-naive or have prior IO experience. In addition, the panel removed category 1 designation from the respective regimens in subsequent therapy, including axitinib [Inlyta], cabozantinib [Cabometyx], nivolumab [Opdivo], and tivozanib [Fotivda]. This is due to the observation by the panel that randomized phase 3 studies for these monotherapies began prior to the approval of immunotherapy combinations and very few patients enrolled in these trials received upfront immunotherapy combinations. Therefore, they don't reflect the current patient we see, as most of the patients have received prior immunotherapy.
The most recent NCCN guidelines look at subsequent therapy for clear philosophy. If patients are immunotherapy naive, there is no preferred regimen and recommended regimens include an immunotherapy combination treatment. If the patient received prior immunotherapy, there is also no preferred regimen due to the lack of phase 3 data, and the recommended regimens in this case are axitinib, cabozantinib, lenvatinib/everolimus, and tivozanib.
Deepak Kilari, MD
Associate Professor
Froedtert & the Medical College of Wisconsin Clinical Cancer Center
Milwaukee, WI

KILARI: If you look at the VEGF tyrosine kinase inhibitors, the drugs are approved based on phase 2 and phase 3 trials. Axitinib was approved on a phase 3 trial [AXIS; NCT00678392], where it was compared with sorafenib [Nexavar]. Tivozanib was compared with sorafenib, as well, and it was in a phase 3 trial [NCT02627963]. In the axitinib trial, I don't think anyone received IO because IO was not approved back then. Lenvatinib plus everolimus had a phase 2 trial [NCT01136733] looking at lenvatinib/everolimus vs everolimus [alone]. Cabozantinib has both retrospective as well as prospective data to support its use in a second-line setting and data from the retrospective study suggesting it is effective even in the third-line setting.
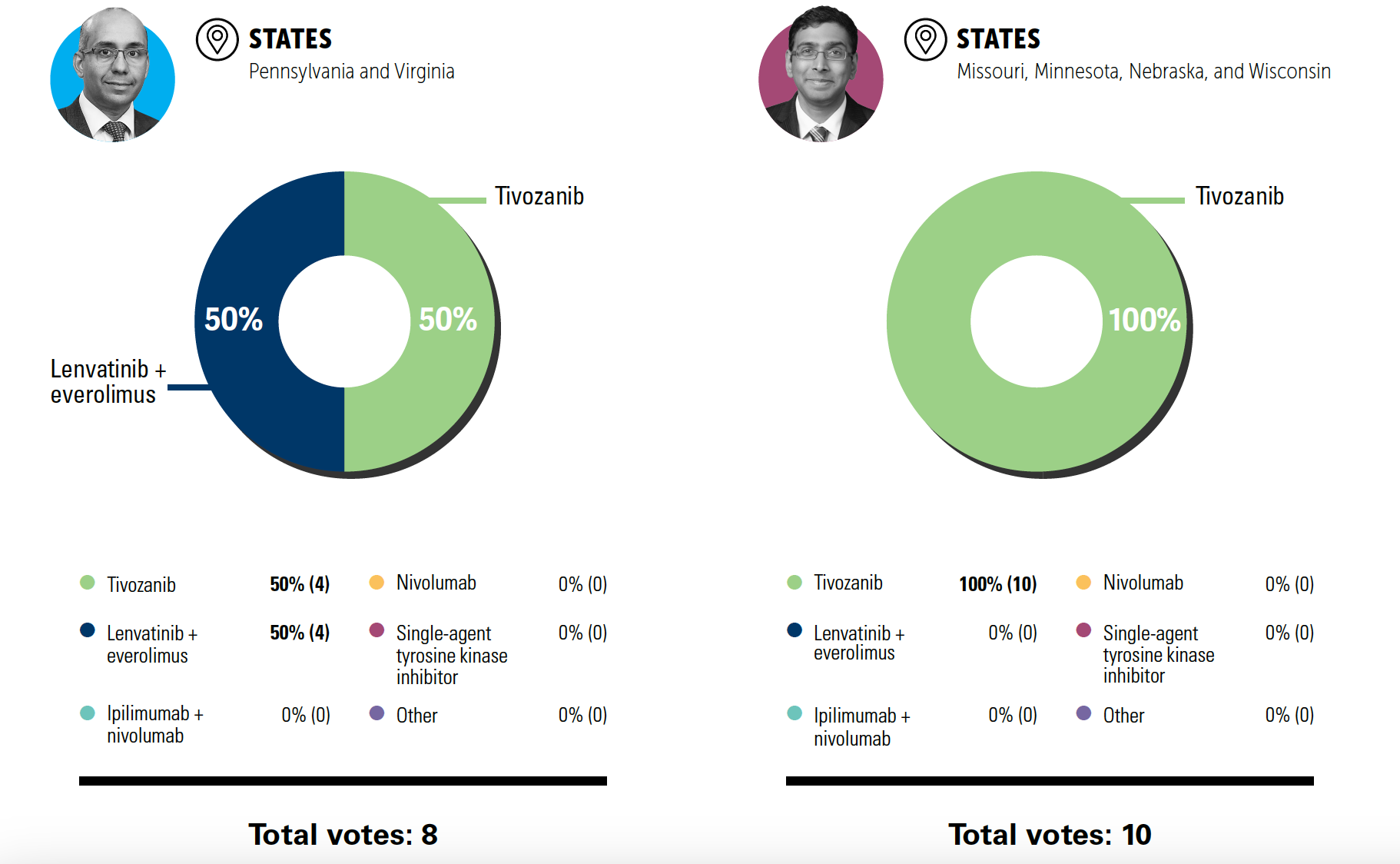
POLLING QUESTION
What are you most likely to recommend for this patient upon disease progression, outside a clinical trial?
REFERENCE
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 1.2024. Accessed November 8, 2023. https://bit.ly/468r8BO

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen