Advancing T-Cell Redirecting Agents in Prostate Cancer
During a conference presentation, A. Oliver Sartor, MD, explained how researchers are building upon the principles of T-cell redirecting agents, such as bispecific T-cell engagers, from other cancer types for the sake of treating patients with prostate cancer.
A. Oliver Sartor, MD

T-cell engaging agents are working their way toward the treatment landscape in prostate cancer with many bispecific antibodies currently in development.
During a presentation at the 28th Annual Prostate Cancer Foundation Scientific Retreat, A. Oliver Sartor, MD, explained how researchers are building upon the principles of T-cell redirecting agents, such as bispecific T-cell engagers (BiTEs), from other cancer types for the sake of treating patients with prostate cancer.1
Bispecific T-Cell Engagers
Sartor, professor of medicine, medical director for the Tulane Cancer Center, and the C. E. and Bernadine Laborde Professor of Cancer Research, Tulane University School of Medicine, pointed to learnings from acute lymphocytic leukemia with blinatumomab (Blincyto) treatment, a BiTE antibody that targets CD19 and CD3, and the first FDA-approved bispecific immunotherapy agent. This agent redirects cytotoxic T cells to eliminate malignant B cells. Blinatumomab leads to T-cell activation and expansion, which promotes circulating tumor cell proliferation, and apoptosis through serial lysis.2 “This really is a new mechanism by which cancer cells can be killed,” Sartor said.
BiTEs directed against prostate-specific membrane antigen (PSMA) and CD3 are being developed for treating prostate cancer, aiming to reach the efficacy seen with blinatumomab. Two such agents in clinical stages of development include pasotuxizumab (AMG 212) and acapatamab (AMG 160). “Both work the same way: it’s taking a T cell, activating it, directing it toward a tumor cell, but now its PSMA,” Sartor said.
Pasotuxizumab is a canonical BiTE molecule with potent in vitro and in vivo cytotoxicity activity. Updated results were published from the first-in-human study (NCT01723475) of the immunotherapy in patients with advanced castration-resistant prostate cancer (CRPC) with prior treatment failure after at least 1 taxane regimen and disease that is refractory to abiraterone (Zytiga) and/or enzalutamide (Xtandi). In patients treated with subcutaneous pasotuxizumab (n = 31), prostatespecific antigen (PSA) reductions of more than 50% were reported in 9 patients at various dose levels. PSA declines of more than 50% were also reported in 3 patients treated with continuous intravenous pasotuxizumab (n = 16), and 2 of these patients had durable responses. However, in the continuous infusion cohort, there were 3 cases of cytokine release syndrome (CRS) noted.3
The developer, Amgen, stopped development of this agent after this proof-of-concept study in favor of acapatamab.
Acapatamab is a half-life extended BiTE immunotherapy agent. In the ongoing first-in-human study (NCT03792841), patients with mCRPC refractory to novel hormonal therapy and failure of 1 to 2 prior taxane regimens or unsuitability for taxanes, were treated with AMG 160 monotherapy or AMG 160 plus pembrolizumab (Keytruda). In the monotherapy cohort, confirmed PSA reductions of at least 30% were reported in 27.6% of patients and unconfirmed responses observed in 11.4%. Partial responses by RECIST criteria were observed in 20% of patients and 53.3% had stable disease. However, 60.5% had grade 2 CRS and 25.6% had grade 3 CRS. A variety of prophylactic mitigation strategies were employed to try to prevent CRS.4
This agent is also currently being explored in combination with enzalutamide, abiraterone, or AMG 404 in patients with mCRPC (NCT04631601).
AMG 509 is a bispecific antibody that is directed against CD3 and STEAP1. The agent contains 2 humanized anti-STEPA1 fragment domains that bind to STEAP1-expressing cells, an anti-CD3 single-chain variable fragment domain that binds to T cells, and a silent Fc domain to extend serum half-life.
This agent is being investigated in an ongoing phase 1 trial in patients with mCRPC (NCT04221542), but clinical data have yet to be reported.
Additional CD3 bidirectional agents in clinical development for prostate cancer include JNJ-63898081, CC-1, and CCW702, all directed against PSMA and CD3; JNJ-78278343, directed against KLK2 and CD3; and JNJ-70218902, directed against TMEFF2 and CD3 (TABLE1).
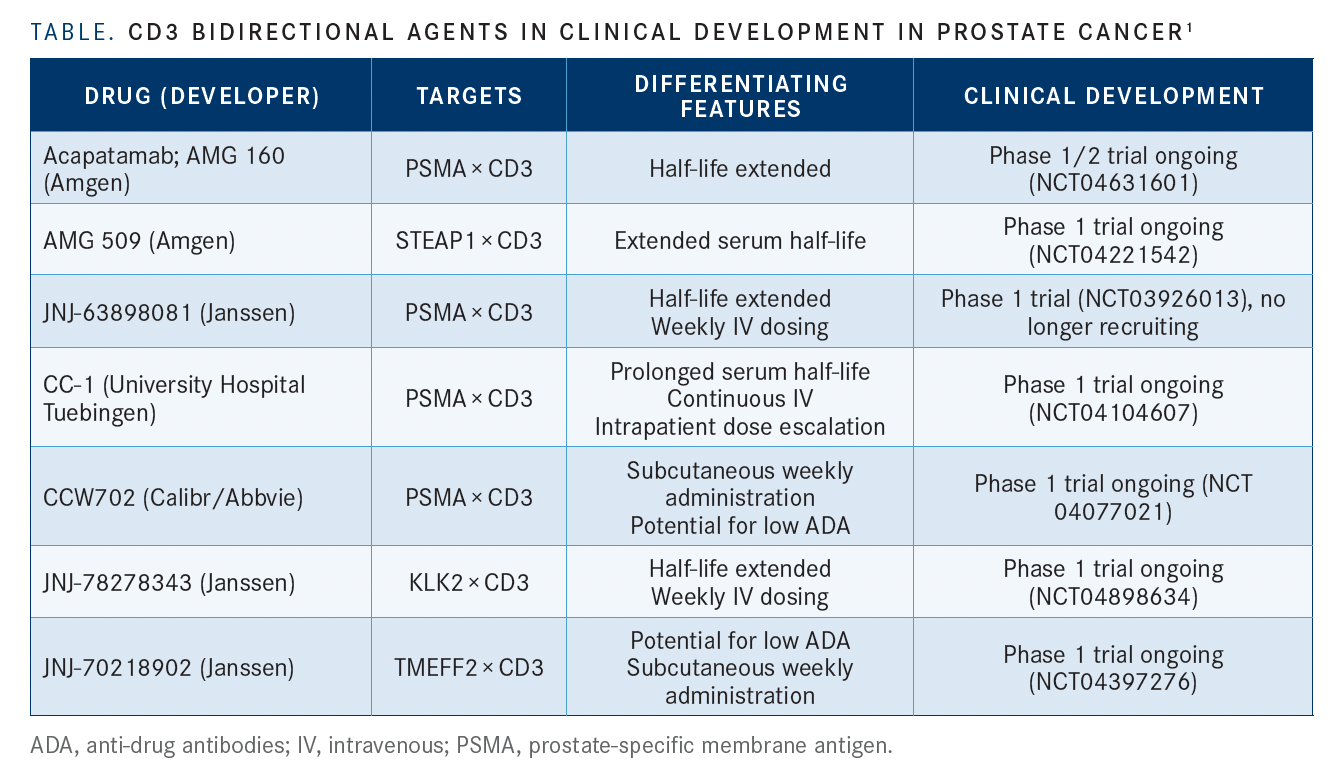
“It is a competitive field…but the interesting thing is there is a variety of different ways to approach the bispecific problem. Which way is best? I don’t know,” Sartor commented.
Multi-specific T-Cell Engagers
HPN424 is a trispecific T-cell engager being investigated for the treatment of prostate cancer. The agent has 3 domains targeted against PSMA for tumor binding, albumin for half-life extension, and CD3 for T-cell engagement. Sartor explained that the agent was constructed to enable solid tumor penetration with prolonged half-life and stability. These features were designed to increase the therapeutic index and minimize off-target toxicities compared with other T-cell engagers.
In the ongoing phase 1/2 first-in-human study (NCT03577028), HPN424 is being investigated in patients with mCRPC who received at least 2 prior systemic therapies. Preliminary results showed 20% of all patients had PSA decreases from baseline; decreases were greater than 50% in 4 patients. Among evaluable patients with measurable disease, 56% had stable disease and reduced disease burden, and 1 patient had a confirmed partial response by RECIST criteria. CRS was reported in 69% of patients, but was grade 3 or higher in only 4%.5 Sartor said that “these are provocative data that needs to be better understood.”
The biotech company TeneoBio is developing multispecific fully human therapeutic antibodies based on transgenic rats.6 Sartor said that this program is highly experimental. Of note, he said, TeneoBio has chosen a unique lower affinity anti-CD3 binding domain, unlike other T-cell engagers, which could result in reduced CRS.
Sartor commented that this is a potential step forward but is dependent on the results of ongoing first-in-human studies (NCT04740034).
Risk With Anti-Drug Antibodies
Sartor highlighted that anti-drug antibodies (ADAs) represent a risk to bispecific program development. Treatment-emergent ADAs are of increasing concern with more complex multi-specific/multi-functional antibodies. He stressed that new methods for early assessment of ADA liabilities are needed along with conducting ADA de-risking studies during development.
REFERENCES:
1. Sartor A. Advancing the Frontiers of T-Cell Redirecting Agents in Prostate Cancer. Presented at: 28th Annual Prostate Cancer Foundation Scientific Retreat; October 28-November 5, 2021; virtual.
2. Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69(12):4941-4944. doi:10.1158/0008-5472.CAN-09-0547
3. Hummel HD, Kufer P, Grüllich C, et al. Pasotuxizumab, a BiTE immune therapy for castration-resistant prostate cancer: Phase I, dose-escalation study findings. Future Med. 2021;13(2):125-141. doi:10.2217/imt-2020-0256
4. Tran B, Horvath L, Dorff T, et al. Interim results from a phase 1 study of AMG 160, a half-life extended (HLE), PSMA targeted, bispecific T cell engager BiTE) immune therapy for metastatic castration-resistant prostate cancer mCRPC. Ann Oncol. 2020;31(suppl 4):S507-S549. doi:10.1016/annonc/annonc275
5. De Bono JS, Fong L, Beer TM, et al. Results of an ongoing phase 1/2a dose-escalation study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager, in patients with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2021;39(suppl 15):5013. doi:10.1200/JCO.2021.39.15_suppl.5013
6. Clarke SC, Ma B, Trinklein ND, et al. Multispecifi c antibody development platform based on human heavy chain antibodies. Front Immunol. 2019;9:3037. doi:10.3389/fi mmu.2018.03037

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More