Zakharia Explains Data Behind Therapeutic Sequencing in mCRPC
During a Targeted Oncology case-based roundtable event, Yousef Zakharia, MD, discussed new data supporting later-line options for patients with metastatic castration-resistant prostate cancer.

Yousef Zakharia, MD
Clinical Associate Professor of Internal Medicine-Hematology, Oncology, and Blood & Marrow Transplantation
University of Iowa Health Care
Iowa City, IA

Targeted OncologyTM: What are the current National Comprehensive Cancer Network (NCCN) guidelines for the use of cabazitaxel (Jevtana) in patients with metastatic castration-resistant prostate cancer (mCRPC)?
ZAKHARIA: The current NCCN guidelines regarding cabazitaxel [say to] use it concurrently with steroids for patients who are not candidates for docetaxel [Taxotere] or who are intolerant to docetaxel.1 Generally speaking, cabazitaxel [can cause] lower peripheral neuropathy compared with docetaxel, especially at the 20-mg/m2 dose. Cabazitaxel is approved for use, post docetaxel, in the second-line setting. The reason that cabazitaxel works after docetaxel is it has poor affinity for MDR, or multidrug [resistance] gene, so it tends to work in the refractory setting after docetaxel.
Cabazitaxel 20 mg/m2 is the usual dose, and GM-CSF [granulocyte-macrophage colony-stimulating growth factor] support will likely be needed. Occasionally, we would use cabazitaxel plus carboplatin with an area under the curve of 4 mg/mL/min, with GM-CSF support for patients who have aggressive variants, especially if they have a certain gene alteration, like PTEN, TP53, or RB1 defects.
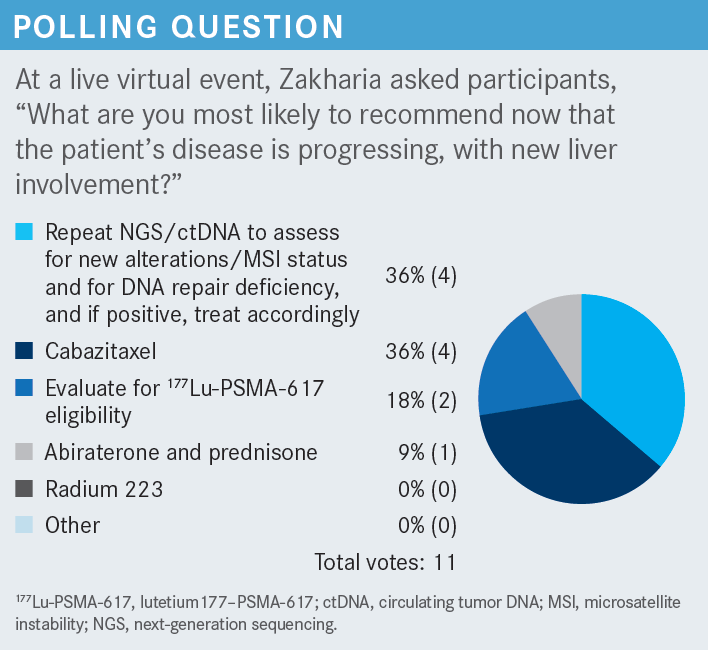
Please discuss the design of the CARD trial [NCT02485691].
[This trial] led to the FDA approval of cabazitaxel, and it was randomization between cabazitaxel vs AR [androgen receptor]–targeted therapy, depending on what [patients] had received in the past.2 So if [the patient] had received abiraterone [Zytiga], they would get enzalutamide, and if they had received enzalutamide, they would go with abiraterone. Now, one might wonder, is this the best competitor arm? We all know about scatter data and literature [on] doing sequencing; AR therapy is not the best strategy. Nevertheless, at the time of this clinical trial, I think those were our treatment options.
Patients with mCRPC who progressed on prior hormonal therapy before or after docetaxel were randomly assigned to either cabazitaxel at 25 mg/m2—this was the first dose, or the higher dose, but other clinical trials established 20 mg/m2 as a preferred dose—vs abiraterone or enzalutamide.2 This was 1:1 randomization with a primary end point of radiographic progression-free survival [rPFS], with key secondary end points of overall survival [OS] and overall PFS, including symptoms or PSA response.
Other secondary end points: pain response, time to symptomatic skeletal event. Stratification factors were based on ECOG performance status, time to progression on prior hormonal therapy—whether within 6 months, or 6 months to 12 months—and timing of the hormonal therapy before or after docetaxel.
The baseline characteristics created a balanced study.2,3 However, [the number of] patients older than 75 years [was] slightly higher in the cabazitaxel arm, at 35% vs 27%. Pain, plus or minus PSA, plus or minus radiographic progression, which was a type of progression at the study entry, was about 66% in the cabazitaxel arm vs 71% in the abiraterone/enzalutamide arm. Gleason score also seems to be slightly higher in the abiraterone/enzalutamide arm, 64% vs 56%, respectively.
The M1 disease at the time of diagnosis, de novo metastatic disease, was slightly higher in the abiraterone/ enzalutamide [arm] compared with the cabazitaxel [arm].2,3
What were the efficacy results of the CARD trial?
The primary end point of rPFS was in favor of cabazitaxel compared with the [AR], with a median image-based PFS of 8 months vs 3.7 months, and that was statistically significant with an HR of 0.54 [95% CI, 0.40-0.73; P < .001].2
In looking at the subgroup analysis across the all-patient subgroups, whether according to performance status or any of the other stratifications, it was overall in favor of the cabazitaxel compared with the abiraterone or enzalutamide.2
In looking at the overall PFS, whether symptomatic or with PSA, along with the radiographic, same thing: It favors the cabazitaxel. Although that difference here, in medium PFS, is not as pronounced, you can see it: 4.4 months in cabazitaxel vs 2.7 months, still statistically significant with an HR of 0.52 in favor of the cabazitaxel [95% CI, 0.40- 0.68; P < .001].2
Then looking at the OS, this was favoring the cabazitaxel, 13.6 months vs 11 months with an HR of 0.64, with a statistically significant P value [95% CI, 0.46- 0.89; P = .008].2
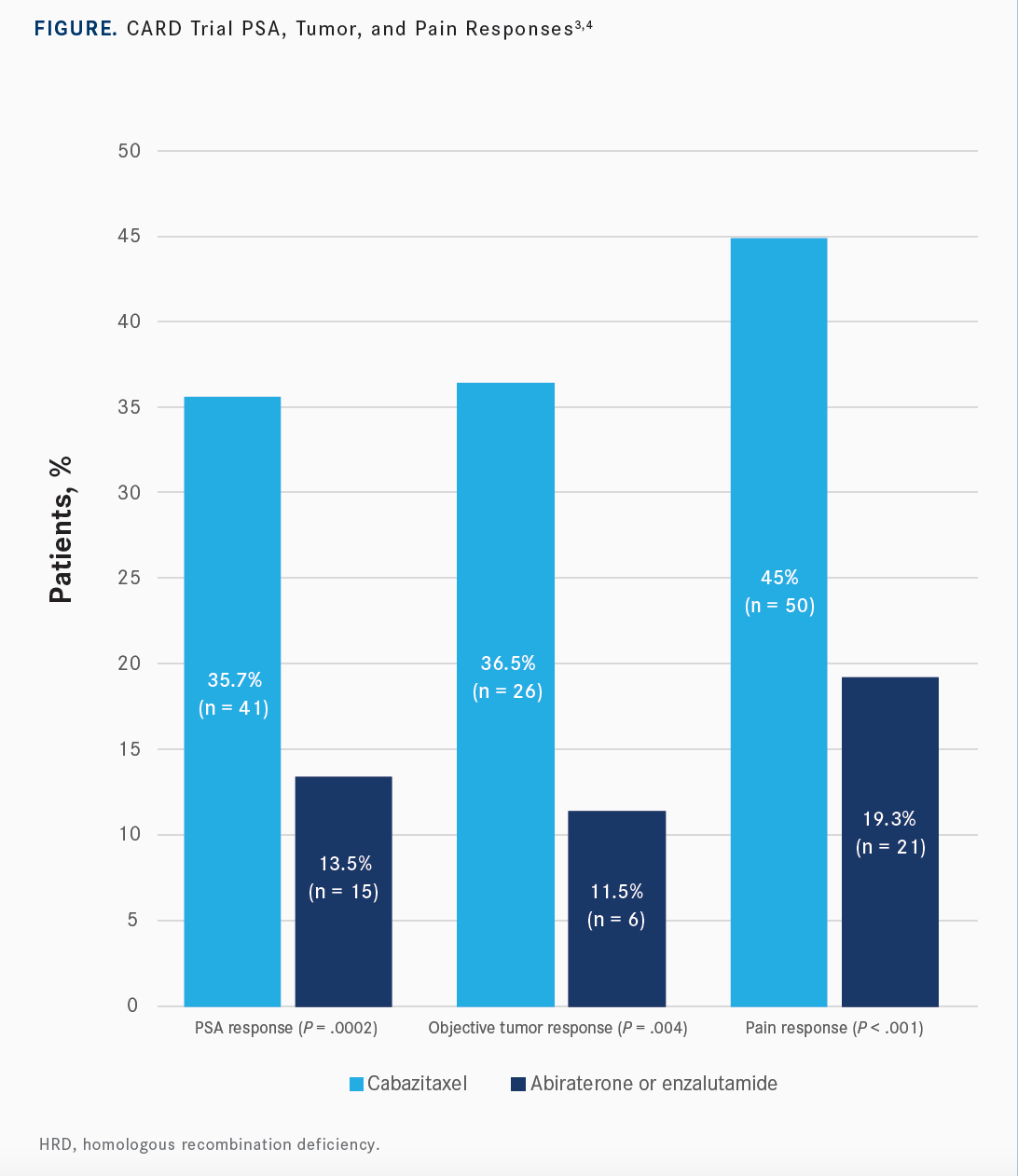
In looking at the different secondary end points, including PSA response, overall response, radiographic response, and in the tumor response, or pain response, all of them were in favor of cabazitaxel over the hormonal therapy [Figure3,4]. The timing to skeletal event was still in favor of the cabazitaxel: median not reached with the cabazitaxel vs 16.7 months in the hormonal therapy.
What were the safety results of this trial?
Adverse events [AEs] leading to treatment discontinuation were more pronounced in the cabazitaxel arm, with almost 20% of AEs leading to treatment discontinuation vs 9% in the abiraterone/enzalutamide arm.2,3 Maybe part of it is the 25 mg/m2, the higher dose, or maybe the patients are more beaten out, so after prior chemotherapy, they might not [have tolerated] further [treatment]. Nevertheless, the data were in favor of 20 mg/m2 on subsequent studies.
In looking at health-related quality of life, using the FACT-P [Functional Assessment of Cancer Therapy– Prostate] survey—which has multiple components to it, including physical well-being, emotional well-being, functional status, and “prostate-specific concerns” well-being—the results still numerically preferred the cabazitaxel over the hormone therapy, but that was not necessarily statistically significant [P = .11].5 However, when looking at the “pain-related subscale,” well-being did favor the cabazitaxel over the hormone therapy [P < .001]. I don’t necessarily find this surprising, again, with the hormonal therapy.
Please discuss the design of the VISION trial [NCT03511664].
[The VISION trial] utilized PSMA [prostate-specific membrane antigen], which is a hot topic now in prostate cancer.6 PSMA is basically an antigen that is specific to prostate cancer cells, along with many other cells in the body, including the salivary gland. So this PSMA, or lutetium targeted therapies, attacking antibody causes local radiation to the prostate cancer cells.
[This study recently] led to the FDA approval of lutetium 177 [177Lu]–PSMA-617 [lutetium Lu 177 vipivotide tetraxetan; Pluvicto].7,8 This was in the CRPC setting, for patients who had received prior androgen therapy and 1 or 2 lines of taxane-based chemotherapy. Patients were randomly assigned to either standard of care and the provider’s choice vs that same standard of care plus 177Lu–PSMA- 617. This was given once every 6 weeks for 4 cycles and [could be increased] up to 6 cycles if the patient was tolerating it. We did have this trial open here at the University of Iowa, and we did enroll a significant number of patients.
As I mentioned, the standard of care was the investigator’s choice, although they did not allow chemotherapy and radium 223 here.6 That left us with the majority of patients getting hormonal therapy, another enzalutamide-after-abiraterone or abiraterone-after-enzalutamide choice, basically. Patients were stratified according to their ECOG performance status, LDH [lactate dehydrogenase] status, [whether they had] liver metastases, and the [AR] pathway inhibitor they received. They were followed every 8 weeks with CT, MRI, or bone scan and then subsequently, after treatment, every 12 weeks.
The primary end point was also rPFS with other secondary end points, including OS.6 In looking at the baseline characteristics, 581 patients were included in this analysis. For all patients who underwent randomization, there were 831 patients. Again, it’s quite well balanced in both arms.
I wanted to highlight that the site of metastases in the rPFS group was quite well balanced in the all-patient population as well; maybe slightly more liver and lung metastases in the standard-of-care arm but not a huge difference.6 About 40% of the patients did have prior cabazitaxel, and almost all of them did have docetaxel, at 97%.
What were the efficacy and safety results of this study?
In looking at the coprimary end points, including the rPFS, the data were in favor of the combination of 177Lu–PSMA-617 plus the standard of care.6 That was statistically significant with a median rPFS of 8.7 months in the combination, vs 3.4 months, with a significant HR of 0.4 [95% CI, 0.29-0.57; P < .001]. The coprimary end point in the all-randomized patients was still in favor of the PSMA, 15.3 months vs 11.3 months [HR, 0.62; 95% CI, 0.52-0.74; P < .001].
In looking at the treatment-emergent AEs, as we all might expect—being local radiation to the prostate cancer metastatic sites, with bone being the most common—the bone marrow suppression is quite a bit overpronounced with this combination.6 About 47% in the PSMA group had bone marrow suppression, with anemia being the most pronounced.
We did encounter a significant amount of patients with leukopenia and thrombocytopenia who had a hard time recovering their counts, in my practice. Dry mouth tends to be quite pronounced with this modality because, remember, PSMA can be expressed in the salivary glands, and even when we do the gallium-PSMA diagnostic test, it lights up in the salivary glands. Nausea and/or vomiting was more pronounced in this group. It’s renally excreted, so about less than 10% had some elevation in their creatinine.
Overall, this led to the FDA approval of the PSMA. Its [brand name is] Pluvicto, and it was approved in March 2022.8 Unfortunately, there’s a huge shortage in the production of this, so until now, we have been unable to give it as a standard of care.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Prostate cancer, version 4.2022. Accessed May 10, 2022.
2. de Wit R, de Bono J, Sternberg CN, et al; CARD Investigators. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506-2518. doi:10.1056/NEJMoa1911206
3. de Wit R, Kramer G, Eymard JC, et al. CARD: randomized, open-label study of cabazitaxel (CBZ) vs abiraterone (ABI) or enzalutamide (ENZ) in metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2019;30(suppl 5):v851-v934. doi:10.1093/annonc/mdz394
4. Fizazi K, Kramer G, Eymard JC, et al. Pain response and health-related quality of life (HRQL) analysis in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving cabazitaxel (CBZ) versus abiraterone or enzalutamide in the CARD study. Poster presented at: Genitourinary Cancers Symposium; February 13-15, 2020; San Francisco, CA. Abstract 16. https:// bit.ly/3zTk3Ih
5. Fizazi K, Kramer G, Eymard JC, et al. Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): an analysis of a randomised, multicentre, open-label, phase 4 study. Lancet Oncol. 2020;21(11):1513-1525. doi:10.1016/ S1470-2045(20)30449-6
6. Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline part I. J Urol. 2021;205(1):14-21. doi:10.1097/ JU.0000000000001375
7. Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
8. FDA approves Pluvicto for metastatic castration-resistant prostate cancer. FDA. March 23, 2022. Accessed October 6, 2022. https://bit.ly/3sbT8Tz

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More