Roundtable Discussion: Savvides Analyzes Role of Hedgehog Inhibitors and Immunotherapy in BCC
During a Targeted Oncology case-based roundtable event, Panayiotis S. Savvides, MD, PhD, MPH, discussed with participants their experiences using hedgehog pathway inhibitors and immunotherapy for patients with basal cell carcinoma.
Panayiotis S. Savvides, MD, PhD, MPH (Moderator)
Medical Oncologist
Mayo Clinic Hospital
Phoenix, AZ


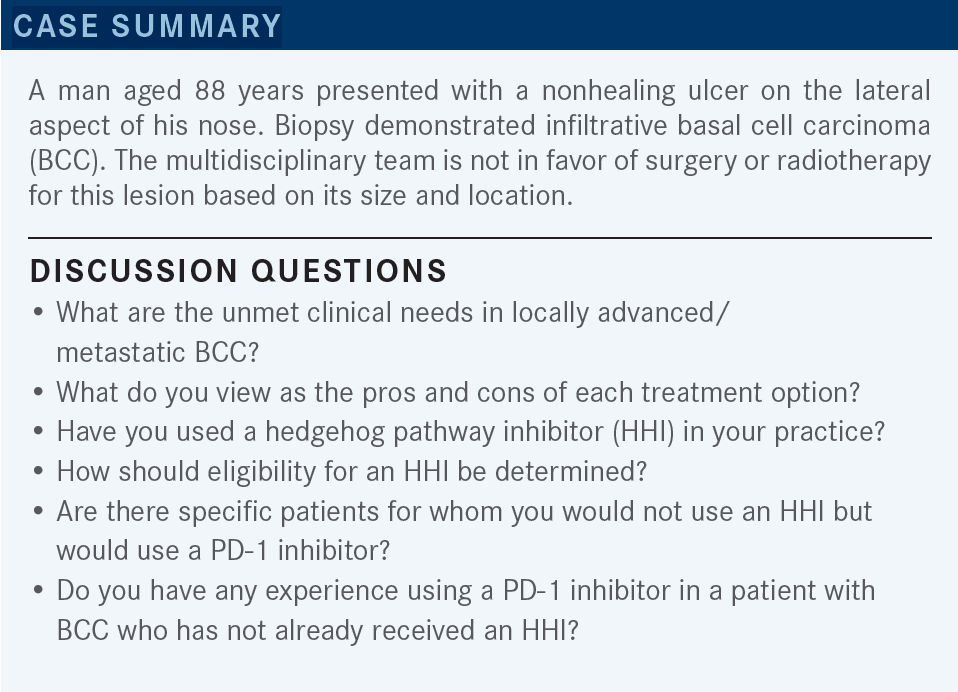
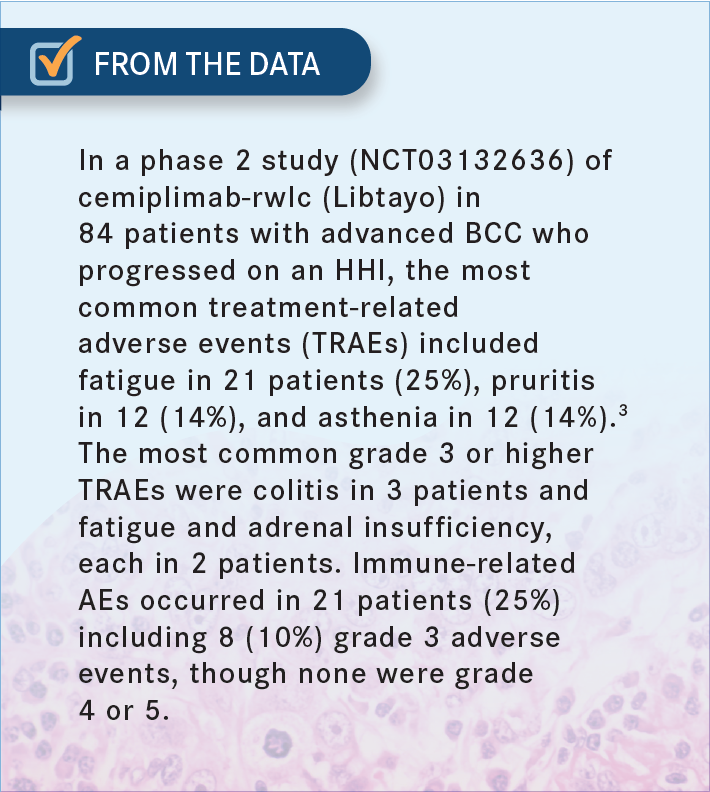
GALAMAGA: I would say one unmet need is how to exploit the hedgehog pathway without the very difficult adverse events [AEs] that some of my older patients report. The muscle aches and myalgias are just intolerable,1,2 so [much so] that some of them down-dose to 1 tablet a week, or along those lines, and it still has some activity—but I think that might be [an unmet need].2 In terms of the pros and cons, immunotherapy seems to be quite well tolerated [From the Data3].
I did have a patient who responded very well to HHIs and then developed panhypopituitarism, and she’s on adrenal support now, so I think these pathways all have those potential toxicities. Most of my patients are 70 years or older. I think the struggle is exploiting the hedgehog pathway. The way I see it is the patients respond amazingly well, but the AEs are just quite difficult.
So I think sequencing it in older adults or allowing other therapies for older adults is a major issue, and my experience in that pathway has been that the myalgias are debilitating for a lot of patients, and it requires a lot of dose reduction, dose skipping, and creative dosing.
SAVVIDES: I think that was very useful, and I think it captures most of the issues that we’re struggling with as far as duration, number of discontinuations, and restarting the treatment. These are the challenges. And at which point do we pull the plug? I think that most of the experience up to now was coming from the period where that was the only good option in our armamentarium. So, as we have been doing in all those settings, we try to maximize the benefit, and obviously we stop and restart. But now with the presence of immunotherapeutic options, we [must] revisit and try to better define our strategy for these patients.
AMBIKA: Is there any head-to-head comparison between immunotherapy and HHI in BCC? Because we know immunotherapy is much better tolerated, and if you can put them in remission or partial remission and use the HHI as a second-line therapy, from a tolerance standpoint, do you think it’s a good strategy?
SAVVIDES: One other thing is that it depends on how we started. If we started with the FDA-approved indications, the way to [switch therapies] has been approved. It’s [less clear] in the setting of patients who have progressed, or are not tolerating [therapy], or they’re not good candidates for HHIs. So one of the challenges is, how do you define these patients? Let’s say at the time of initiation of treatment, who would be the HHI-ineligible patient?
This is not so clearly defined. It’s one thing to start the treatment, try to see how well it will be tolerated, and then move to the next line of treatment once you run into toxicity or efficacy problems. It’s different to [do this] up front. I don’t think that we have data to clearly say, unless we have some experience that we think that this patient will not be able to tolerate it—patients with preexisting GI [gastrointestinal] symptoms, frail patients, [where] we think that [treatment intolerance] might be overt—it will be hard to, a priori, make that decision, [because] most of the population is elderly. I think it also depends on our experience.
To be honest, I’ve been somehow surprised, because even in dermatology practices, where they have a lot of HHI use and experience, it seems that the younger practitioners are using immunotherapeutic options more frequently as a first-line therapy because they’re more comfortable with that, as opposed to their more senior colleagues. So I think that it’s also a cultural issue as our experience and level of comfort with both treatment modalities increases with time.
GONZALEZ-VELEZ: I will say that an unmet need would be for solid organ transplant patients in which you have more difficulties or contraindication against using the immunotherapy, [as well as] the HHI having difficult toxicity.
SAVVIDES: That’s a very good point because obviously, in that patient population, any use of immunotherapy at this point is clearly not standard of care. Obviously, there is an unmet need there for additional options in that setting, but at this point that is correct.
I think that outside of a clinical trial, and especially outside of a specialized center, it’s hard to try any therapeutic approach in that patient population, because the risk is too high. Have you used cemiplimab or a checkpoint inhibitor in a [patient with] BCC…who has not already received an HHI?
OLIVER: I had one patient who got immunotherapy because he was too frail. He had a remote history of liver transplant. He did…well on it, and he tolerated it well. He got a good 3 to 3.5 years from it, so he had a great response.

SAVVIDES: What are the goals of therapy, as far as setting expectations, tolerating toxicity, modifying the dose, and treatment breaks?
GALAMAGA: I would say we continue to [treat until] best response in our patients, and I’ve had dramatic results in several patients. The myalgias eventually do affect therapy, and if those patients are not good candidates for surgery, we are trying to offer them the longest-term therapy with the highest degree of disease control. I even had one patient [who] took 1 dose per week and still had AEs, so I’ve resorted to off-label dosing strategies to help some of these patients get through it.
SAVVIDES: And was there any extreme match as far as age-wise, because I guess you can expect that the metabolism might be different with more advanced age, or was it just your garden variety as far as age?
GALAMAGA: My patient was [in their] mid-80s, so I’m sure age and metabolic factors play a role.
SAVVIDES: How do you [address] quality-of-life issues as you move along with treatment and frequency of duration of treatment breaks?
GONZALEZ-VELEZ: For quality of life, there are different modifications, but you can do intermittent therapy, or once you achieve a complete response, you can hold it and discontinue the treatment, and then reinitiate the treatment at relapse.2,4 But I think there [are] no specific rules for doing 3 months of treatment, after maybe 2-month breaks, but again, I think there are no specific guidelines for those dosing modifications.
SAVVIDES: I think that there would be a more pressing need to develop these alternative approaches, once that was your only treatment option. The question is, these days, when you have the option of moving to immunotherapy earlier on, because it’s an FDA-approved indication, do you still feel the need to maximize and try to develop an alternative.
REFERENCES
1. Low JA, de Sauvage FJ. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol. 2010;28(36):5321-5326. doi:10.1200/JCO.2010.27.9943
2. Lear JT, Dummer R, Guminski A. Using drug scheduling to manage adverse events associated with hedgehog pathway inhibitors for basal cell carcinoma. Oncotarget. 2021;12(26):2531-2540. doi:10.18632/oncotarget.28145
3. Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):848-857. doi:10.1016/S1470-2045(21)00126-1
4. Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18(3):404-412. doi:10.1016/S1470-2045(17)30072-4