Weighing IO Plus TKI Combinations in Advanced RCC
During a Targeted Oncology™ Case-Based Roundtable™ event, Sandy Liu, MD, discussed the potency of immunotherapy and tyrosine kinase inhibitor combinations for patients with metastatic renal cell carcinoma.
Sandy Liu, MD
Assistant Clinical Professor, Department of Medical Oncology & Therapeutics Research
Medical Director, Division of Genitourinary
City of Hope Orange County
Duarte, CA

At a live virtual event, Sandy Liu, MD, discussed the potency of immunotherapy and tyrosine kinase inhibitor combinations for patients with metastatic renal cell carcinoma. These combinations as first-line therapy have continued to show their superiority over sunitinib monotherapy and have given clinicians a variety of treatment options for this patient population. By having a variety of choice, they can craft individualized treatment plans. However, Liu discusses the different advantages and disadvantages of these combinations and how they fit together in this treatment landscape.
CONSIDERING PATIENTS’ DISEASE RISK STATUS
LIU: Looking at the [National Comprehensive Cancer Network (NCCN) guidelines for] patients with poor/intermediate risk, all the immunotherapy [IO] plus tyrosine kinase inhibitor [TKI] combinations are NCCN category 1 approved [ for this patient population].1 These include axitinib [Inlyta] plus pembrolizumab [Keytruda], cabozantinib [Cabometyx] plus nivolumab [Opdivo], and lenvatinib [Lenvima] plus pembrolizumab. However, I hardly ever use the International Metastatic RCC Database Consortium [IMDC] risk criteria anymore, because we have all the regimens already approved for these groups. I mainly use it for prognostic reasons and clinical trial enrollment.
A lot of clinicians will favor axitinib plus pembrolizumab [in the frontline setting] just because of the adverse event [AE] profile, but some will still choose nivolumab plus ipilimumab. [When using cabozantinib], 40 mg is usually well tolerated, but for some patients, you have to go down to 20 mg [so they tolerate it better]. Cabozantinib plus nivolumab is my preferred [choice in the frontline setting], but I have used all the regimens.
How I approach [frontline treatment for a] newly diagnosed patient is I look at their performance status, comorbidities, site of disease, burden of disease, and [whether] they are symptomatic. I also look at [whether] they can afford to go to second-line therapy. [However], if I have 1 shot at treatment, I don’t think I would choose nivolumab plus ipilimumab. The only reason why is because it has a high progressive disease [PD] rate.2 [This combination] has [approximately] a 20% PD rate, whereas the current IO plus TKI combinations have a much lower PD rate [across trials]. Again, if I have 1 shot at treatment, I will use an IO and TKI combination.
I also have been doing active surveillance for patients [who] have small pulmonary nodules, a very low disease burden, and/or are asymptomatic, and I watch them closely. Most patients eventually start treatment at [approximately] 14 months, but it hasn’t changed their survival outcomes…. In patients with low burden disease, I do active surveillance until I see the pace of the disease picking up.3
LENVATINIB PLUS PEMBROLIZUMAB’S ROLE IN TREATMENT
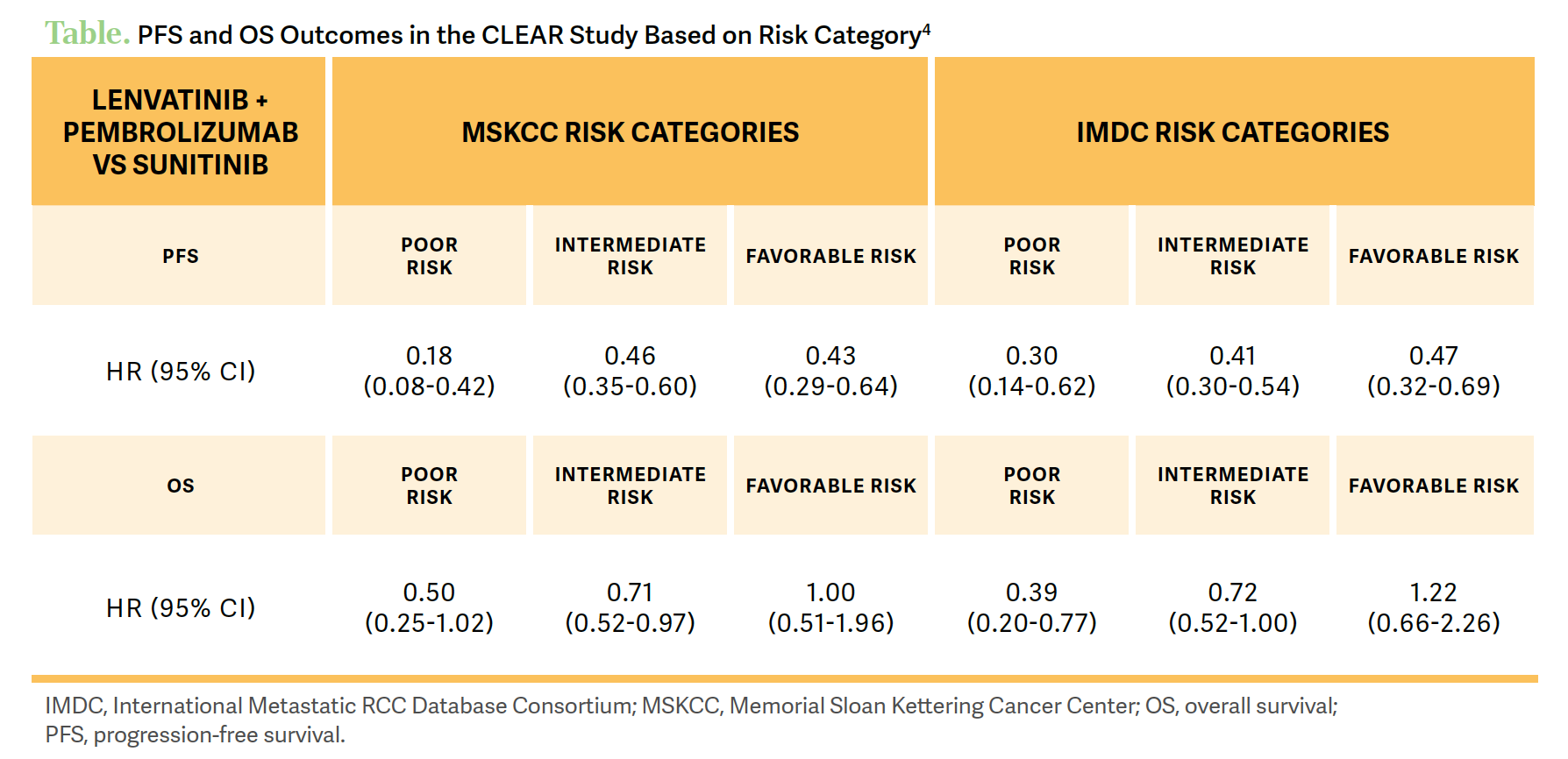
The [phase 3 CLEAR study (NCT02811861)] showed a progression-free survival [PFS] benefit for patients [who received] lenvatinib plus pembrolizumab at a median follow-up of 33.7 months.4 There was a significant PFS benefit for the combination therapy compared with patients on sunitinib, at a median PFS of 23.3 months [95% CI, 20.8-27.7] vs 9.2 months [95% CI, 6.0-11.0], respectively [HR, 0.42; 95% CI, 0.34-0.52]. [Investigators] also saw a PFS benefit across all IMDC and Memorial Sloan Kettering Cancer Center risk groups. They also observed an overall survival [OS] benefit across poor- and intermediate-risk patient groups, and the favorable-risk group still had a trend toward survival [Table4].
What was notable in the response rates of this trial, at the time of its final analysis, was the high objective response rate [ORR] for patients on lenvatinib plus pembrolizumab, at 71.3% [95% CI, 66.6%-76.0%].5 This is the highest response rate of any IO plus TKI combination compared [with] the ORR of patients on sunitinib, at 36.7% [95% CI, 31.7%- 41.7%]. What was even more impressive is the complete response [CR] rate, at 18% compared [with] approximately 4% with sunitinib. One out of 6 patients had a CR, and the PD rate for this combination was pretty low, at 5%.5
TOXICITIES WITH IO/TKI COMBINATIONS
Regarding the toxicities, grade 3 or greater AEs with lenvatinib plus pembrolizumab occurred in 82.4% of patients. [Approximately] 69% of patients had to have their dose of lenvatinib reduced, and [approximately] 26% had to discontinue lenvatinib on this study compared [with] 14% of patients on sunitinib. So, you do see a high discontinuation rate from lenvatinib.5
However, for my younger patients who need a quick response, this is the combination I go to because of the high response rate and high CR rate. Again, there [are] a lot of AEs [with lenvatinib and pembrolizumab], but if you do appropriate dose reduction and dose interruption, you can keep patients on treatment longer.6 Some clinicians I know start low then go high [with a patient’s dose of lenvatinib to manage these toxicities].
Overall, the TKI toxicity profiles are very similar [across the drug type], but I notice a little more fatigue and hypertension with lenvatinib. I do see more diarrhea with axitinib,7 but with cabozantinib and nivolumab, I see a little more liver toxicity.8 So [there are] just some slight differences regarding toxicities.
UPDATED DATA CONTINUE TO SUPPORT IO/TKI TREATMENT
[Updated data from the CheckMate 9ER trial (NCT03141177) at] a median 44-month [range, 36.5-56.5] follow-up [were] presented at the 2023 American Society of Clinical Oncology Genitourinary Cancers Symposium.9 At 44 months, this is the longest IO and TKI combination that has been reported out, and there is still a PFS benefit for patients on nivolumab plus cabozantinib vs sunitinib monotherapy, at 16.6 months [95% CI, 12.8-19.8] vs 8.4 months [95% CI, 7.0-9.7], respectively [HR, 0.58; 95% CI, 0.48-0.71; P < .0001]. At almost 4 years of follow-up, there is a doubling of the PFS with the combination therapy compared [with] sunitinib.
For OS at the 44-month follow-up, there was again a significant improvement in median OS for patients on nivolumab and cabozantinib vs those on sunitinib, at 49.5 months [95% CI, 40.3-not estimable] compared with 35.5 months [95% CI, 29.2-42.3], respectively [HR, 0.70; 95% CI, 29.2-42.3; P = .0014]. Again, these results remain consistent since the primary analysis at 18 months. [Investigators] also looked at PFS and OS by IMDC risk group at the 44-month follow-up. For patients with favorable, intermediate, and poor risk, there is a survival benefit [with] nivolumab and cabozantinib across all risk groups.9
Lastly, there was the KEYNOTE-426 trial [NCT02853331], which looked at pembrolizumab and axitinib against sunitinib monotherapy. It was a similar study design to the other IO and TKI combination trials. Patients were [randomly assigned] 1:1 to either 250 mg of pembrolizumab given intravenously every 3 weeks and 5 mg of axitinib given orally twice a day, compared with patients on 50 mg of sunitinib given orally twice a day for the first 4 weeks of each 6-week cycle.10
The primary end point of KEYNOTE-426 was OS and PFS. At a median follow-up of 42.8 months, there was an OS benefit in the intent-to-treat population of patients on the combination of pembrolizumab and axitinib. The median OS for this group was 45.7 months [95% CI, 43.6–not reached] compared with 40.1 months [95% CI, 34.3-44.2] for patients on sunitinib, with an HR of 0.73 [95% CI, 0.60-0.88; P < .001].11
References
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 1.2024. Accessed August 9, 2023. https://tinyurl.com/h2y8hfef
2. Motzer RJ, Tannir NM, McDermott DF, et al. Conditional survival and 5-year follow-up in CheckMate 214: first-line nivolumab + ipilimumab (N+I) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). Ann Oncol. 2021;32(suppl 5):S678-S724. doi:10.1016/annonc/annonc675
3. Harrison MR, Costello BA, Bhavsar NA, et al. Active surveillance of metastatic renal cell carcinoma: results from a prospective observational study (MaRCC). Cancer. 2021;127(13):2204-2212. doi:10.1002/cncr.33494
4. Porta C, Eto M, Motzer R, et al. Updated efficacy of lenvatinib (LEN) + pembrolizumab (PEMBRO) vs sunitinib (SUN) in patients (pts) with advanced renal cell carcinoma (aRCC) in the CLEAR study. Ann Oncol. 2022;33(suppl 7):S660-S680. doi:10.1016/annonc/annonc1072
5. Motzer RJ, Powles T, Hutson T, et al. Characterization of tumor response with lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma: final overall survival analysis of the CLEAR study (4-year median follow up). Presented at: 2023 Kidney Cancer Research Summit; July 13-14, 2023; Boston, MA. Abstract 66.
6. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
7. Rini BI, Atkins MB, Choueiri TK, et al. Time to resolution of axitinib-related adverse events after treatment interruption in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2021;19(5):e306-e312. doi:10.1016/j.clgc.2021.03.019
8. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
9. Burotto M, Powles T, Escudier B, et al. Nivolumab plus cabozantinib vs sunitinib for first-line treatment of advanced renal cell carcinoma (aRCC): 3-year follow-up from the phase 3 CheckMate 9ER trial. J Clin Oncol. 2023;41(suppl 6):603. doi:10.1200/JCO.2023.41.6_suppl.603
10. Powles T, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for locally advanced or metastatic renal cell carcinoma (mRCC): phase III KEYNOTE-426 study. J Clin Oncol. 2019;37(suppl 7):543. doi:10.1200/JCO.2019.37.7_suppl.543
11. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): results from 42-month follow-up of KEYNOTE-426. J Clin Oncol. 2021;39(suppl 15):4500. doi:10.1200/JCO.2021.39.15_suppl.4500

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen