Updated KEYNOTE-048 Data Show PD-1 Inhibitor Improves OS in HNSCC
Updated results of the phase III KEYNOTE-048 trial comparing pembrolizumab as monotherapy and in combination with platinum chemotherapy and fluorouracil with standard-of-care chemotherapy support the use of the PD-1 inhibitor in the frontline for patients with recurrent or metastatic head and neck squamous cell carcinoma, according to Danny Rischin, MD, who presented the results of the final analysis at the 2019 American Society of Clinical Oncology Annual Meeting.
Danny Rischin, MD
Updated results of the phase III KEYNOTE-048 trial comparing pembrolizumab (Keytruda) as monotherapy and in combination with platinum chemotherapy and fluorouracil (5-FU) with standard-of-care chemotherapy support the use of the PD-1 inhibitor in the frontline for patients with recurrent or metastatic head and neck squamous cell carcinoma, according to Danny Rischin, MD, who presented the results of the final analysis at the 2019 American Society of Clinical Oncology Annual Meeting.1
In the overall population, pembrolizumab and chemotherapy reduced the risk of death by 28% versus cetuximab (Erbitux) plus platinum chemotherapy and 5-FU, known as the EXTREME regimen, in all patients regardless of PD-L1 status. In patients with a combined positive score (CPS) ≥20 receiving pembrolizumab monotherapy, investigators observed a 42% reduction in the risk of death versus the control.
“Pembrolizumab when combined with platinum and 5-FU compared with EXTREME resulted in superior overall survival [OS] in the PD-L1 CPS ≥20, CPS ≥1, and total populations. The duration of response [DOR] was longer, and the safety profile was comparable between the 2 regimens,” said Rischin, director of the Division of Cancer Medicine and head of the Department of Medical Oncology at Peter MacCallum Cancer Centre in Melbourne, Australia. “With regard to pembrolizumab monotherapy when compared with EXTREME, [the agent] resulted in superior OS in the PD-L1 CPS ≥20 and ≥1 populations, and it was noninferior…in the total population. The DOR was substantially longer with pembrolizumab, and the safety profile was favorable compared with EXTREME.”
At the European Society for Medical Oncology 2018 Congress, data presented by Barbara Burtness, MD, co-director of the Developmental Therapeutics Research Program and professor of medicine (medical oncology) at Yale Cancer Center in New Haven, Connecticut, showed that pembrolizumab was superior to the EXTREME regimen for OS in the CPS ≥20 population and that the hazard ratio for death for this patient population was 0.61, which was considered to be highly statistically significant. The median OS with pembrolizumab was 14.9 months versus 10.7 months in the control arm. “This happened despite the fact that pembrolizumab does not lead to as high a response rate and had a somewhat shorter PFS [progression-free survival],” she said in an interview withTargeted Therapies in Oncology. Of note, the difference in PFS for this population was not statistically significant (HR, 0.99;P= .5).2
The data cutoff for the updated analysis was February 25, 2019. For eligibility, patients had to be naïve to chemotherapy or systemic therapy for recurrent or metastatic squamous cell carcinoma of the oral cavity, the oropharynx, the larynx, or the hypopharynx; have an ECOG performance status of ≤1; and have a PD-L1 biomarker analysis for stratification. Patients were randomized 1:1:1 to 3 comparator arms, either single-agent pembrolizumab, pembrolizumab with platinum chemotherapy and 5-FU, or the standard-of-care EXTREME regimen (FIGURE).
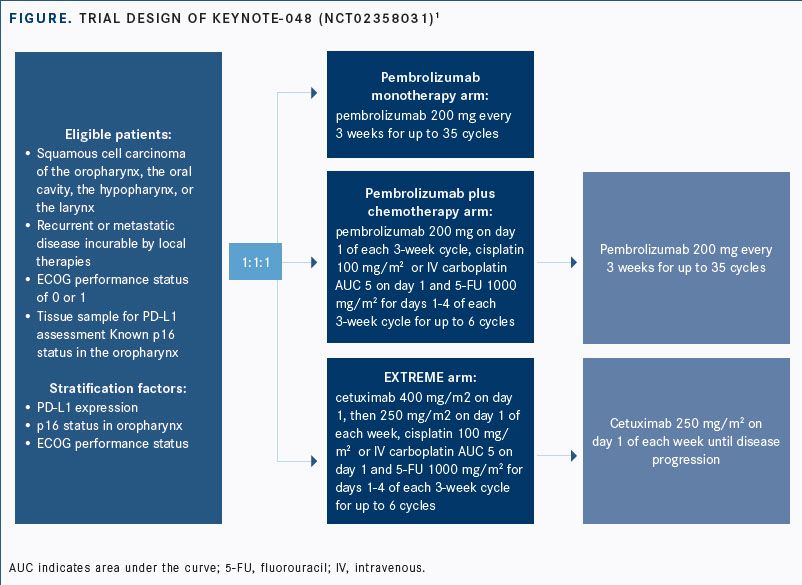
The study’s primary endpoints were PFS and OS in the overall population and in subgroups with CPS ≥20 and ≥1. Secondary endpoints included objective response rate (ORR), rates of PFS, quality of life, and safety. DOR in the total cohort and key subgroups by PD-L1 status served as an exploratory endpoint.
Baselines characteristics in the intention-to-treat population were balanced between the pembrolizumab monotherapy and EXTREME arms as well as between pembrolizumab plus chemotherapy versus EXTREME. The majority of patients had an ECOG performances status of 1, were male, and were current or former smokers. By PD-L1 status, 85.3% of patients had a CPS ≥1, and 42.3% had a CPS ≥20.
Pembrolizumab Plus Chemotherapy Versus EXTREME
In the comparison between pembrolizumab plus chemotherapy versus EXTREME in the CPS ≥20 group, updated OS analysis showed that the immunotherapy combination improved median OS at 14.7 months versus 11.0 months with the standard of care (HR, 0.60; 95% CI, 0.45-0.82;P= .0004). The rates of PFS at 12 and 24 months were 57.1% and 35.4% with the pembrolizumab combination, respectively, versus 46.1% and 19.4% with EXTREME. Median PFS in this group was 5.8 months for pembrolizumab and chemotherapy versus 5.2 months with EXTREME, but these results did not reach the threshold for superiority (HR, 0.73; 95% CI, 0.55-0.97;P= .0162).
For patients with CPS ≥1, corresponding rates of median OS were 13.6 months and 10.4 months with pembrolizumab plus platinum and EXTREME (HR, 0.65; 95% CI, 0.53-0.80;P<.0001). Rates of PFS at 12 months were 55.0% versus 43.5% with the pembrolizumab combination and EXTREME, respectively; at 24 months, the rates were 30.8% and 16.8%. The difference in PFS for this group was not statistically significant.
Response rates were similar between these regimens. In the CPS ≥20 group, the ORR was 42.9% for patients receiving the pembrolizumab-containing regimen versus 38.2% with EXTREME. For patients with CPS ≥1, the corresponding rates of ORR were 36.4% and 35.7%, respectively.
The median DOR for patients with CPS ≥20 was 7.1 months versus 4.2 months with chemoimmunotherapy and EXTREME, respectively. For patients with CPS ≥1, the corresponding median DOR was 6.7 months versus 4.3 months.
In the overall study population, pembrolizumab plus chemotherapy improved median OS (13.0 vs 10.7 months; HR, 0.72; 95% CI, 0.60- 0.87), and this was found to be significant at the interim analysis.
Adverse events (AEs) from any cause were similar between the 2 regimens. Almost all patients receiving the pembrolizumab-containing regimen (98.2%) and EXTREME (99.7%) experienced any-grade AEs, with 85.1% and 83.3% of those experiencing grade ≥3 AEs, respectively. AEs led to discontinuation in 32.6% of patients taking pembrolizumab plus platinum and 5-FU and in 27.5% receiving EXTREME.
Pembrolizumab Monotherapy Versus EXTREME
In the comparison between pembrolizumab monotherapy versus EXTREME in the CPS ≥20 group, the PD-1 inhibitor improved median OS to 14.8 months from 10.7 months with the cetuximab-containing regimen (0.58; 95% CI, 0.44-0.78). Rates of 12- and 24-month PFS were 56.4% and 35.3% with pembrolizumab and 44.9% and 19.1% with EXTREME, respectively.
For patients with CPS ≥1, corresponding rates of median OS were 12.3 months and 10.3 months with pembrolizumab and EXTREME (HR, 0.74; 95% CI, 0.61-0.90). Rates of PFS at 12 months were 50.4% versus 43.6% with pembrolizumab and EXTREME, respectively; at 24 months, the rates were 28.9% and 17.4%.
In the total population, pembrolizumab did not have a statistically significant impact on OS (HR, 0.83; 95% CI, 0.70-0.99; P = .0199), and the ORR was lower than that of EXTREME (16.9% vs 36.0%). However, median DOR was significantly longer with pembrolizumab, at 22.6 months versus 4.5 months observed with EXTREME.
The percentage of all-cause AEs was less with pembrolizumab monotherapy (all-grade, 96.7%; grade ≥3, 54.7%) compared with EXTREME (all-grade, 99.7%; grade ≥3, 83.3%), and fewer patients discontinued therapy with the immunotherapy agent (12.0% vs 27.5%, respectively).
References:
- Rischin D, Harrington KJ, Greil R, et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol. 2019;37(suppl 15; abstr 6000). doi: 10.1200/JCO.2019.37.15_suppl.6000.
- Burtness B, Harrington KJ, Greil R, et al. KEYNOTE-048: phase 3 study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Presented at: European Society of Medical Oncology 2018 Congress; October 19-23, 2018; Munich, Germany. Abstract LBA8_PR. bit.ly/2HY1hp9.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More